Abstract
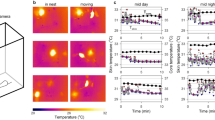
We report on in vivo temperature measurements performed in mice at two specific sites of interest in the animal body over a period of several hours. In particular, the aim of this work was to monitor mouse metabolism during cold exposure, and to record possible temperature differences between the body temperature measured in the abdomen and the temperature of the brown adipose tissue (BAT) situated in the interscapular area. This approach is of biological interest as it may help unravelling the question whether biochemical activation of BAT is associated with local increase in metabolic heat production. For that purpose, miniaturized thermistor sensors have been accurately calibrated and implanted in the BAT and in the abdominal tissue of mice. After 1 week of recovery from surgery, mice were exposed to cold (6 °C) for a maximum duration of 6 h and the temperature was acquired continuously from the two sensors. Control measurements with a conventional rectal probe confirmed good performance of both sensors. Moreover, two different mouse phenotypes could be identified, distinguishable in terms of their metabolic resistance to cold exposure. This difference was analyzed from the thermal point of view by computational simulations. Our simple physical model of the mouse body allowed to reproduce the global evolution of hypothermia and also to explain qualitatively the temperature difference between abdomen and BAT locations. While with our approach, we have demonstrated the importance and feasibility of localized temperature measurements on mice, further optimization of this technique may help better identify local metabolism variations.





Similar content being viewed by others
References
M. D. Alexander, K. T. B. MacQuarrie, Ground Water Monit. Remediat. 25, 75 (2005)
D. D. Bae, P. L. Brown, E. A. Kiyatkin, Brain Res. 1154, 61 (2007)
K. C. Bicego, R. C. H. Barros, L. G. S. Branco, Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 147, 616 (2007)
C. M. L. Burnett, J. L. Grobe, Am. J. Physiol. Endocrinol. Metab. 305, E916 (2013)
C. M. L. Burnett, J. L. Grobe, Mol. Metab. 3, 460 (2014)
C. Cohade, M. Osman, H. K. Pannu, R. L. Wahl, J. Nucl. Med. 44, 170 (2003)
B. Conti, M. Sanchez-Alavez, R. Winsky-Sommerer, M. C. Morale, J. Lucero, S. Brownell, V. Fabre, S. Huitron-Resendiz, S. Henriksen, E. P. Zorrilla, L. de Lecea, T. Bartfai, Science 314, 825 (2006)
J. D. Crane, E. P. Mottillo, T. H. Farncombe, K. M. Morrison, G. R. Steinberg, Mol. Metab. 3, 490 (2014)
R. G. da Silva, A. S. Campos Maia, Principles of Animal Biometeorology (Springer, 2013)
S. DeBow, F. Colbourne, Methods 30, 167 (2003)
F. A. Duck, Physical Properties of Tissues a Comprehensive Reference Book (Academic Press, London, 1990)
M. L. Gantner, B. C. Hazen, J. Conkright, A. Kralli, Proc. Natl. Acad. Sci. U. S. A. 111, 11870 (2014)
S. Gatti, J. Beck, G. Fantuzzi, T. Bartfai, C. A. Dinarello, Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R702 (2002)
C. J. Gordon, J. Therm. Biol. 34, 213 (2009)
C. J. Gordon, J. Therm. Biol. 37, 654 (2012)
M. J. Harms, J. Ishibashi, W. Wang, H.-W. Lim, S. Goyama, T. Sato, M. Kurokawa, K.-J. Won, P. Seale, Cell Metab. 19, 593 (2014)
IUPS Thermal Commission, J. Therm. Biol. 28(75) (2003)
N. Kataoka, H. Hioki, T. Kaneko, K. Nakamura, Cell Metab. 20, 346 (2014)
E. M. Knight, T. M. Brown, S. Gümüsgöz, J. C. M. Smith, E. J. Waters, S. M. Allan, C. B. Lawrence, Dis. Model. Mech. 6, 160 (2013)
D.M. Lateef, G. Abreu-Vieira, C. Xiao, M.L. Reitman, Am. J. Physiol. Endocrinol. Metab. E681 (2014)
J. A. Levine, Public Health Nutr. 8, 1123 (2005)
P. Lomax, Nature 210, 854 (1966)
L. E. Mount, J. Physiol. 217, 315 (1971)
J. Nedergaard, B. Cannon, Cell Metab. 11, 268 (2010)
J. Nedergaard, T. Bengtsson, B. Cannon, Am. J. Physiol. Endocrinol. Metab. 293, E444 (2007)
S. Poole, J. D. Stephenson, Physiol. Behav. 18, 203 (1977)
M. Saito, Obes. Res. Clin. Pract. 7, e432 (2013)
M. Saito, Y. Okamatsu-Ogura, M. Matsushita, K. Watanabe, T. Yoneshiro, J. Nio-Kobayashi, T. Iwanaga, M. Miyagawa, T. Kameya, K. Nakada, Y. Kawai, M. Tsujisaki, Diabetes 58, 1526 (2009)
M. Sanchez-Alavez, S. Alboni, B. Conti, Age Dordr. Neth. 33, 89 (2011)
J. R. Speakman, Integr. Physiol. 4, 34 (2013)
J. S. Steinhart, S. R. Hart, Deep Sea Res. Oceanogr. Abstr. 15, 497 (1968)
M. H. Tschöp, J. R. Speakman, J. R. S. Arch, J. Auwerx, J. C. Brüning, L. Chan, R. H. Eckel, R. V. Farese Jr., J. E. Galgani, C. Hambly, M. A. Herman, T. L. Horvath, B. B. Kahn, S. C. Kozma, E. Maratos-Flier, T. D. Müller, H. Münzberg, P. T. Pfluger, L. Plum, M. L. Reitman, K. Rahmouni, G. I. Shulman, G. Thomas, C. R. Kahn, E. Ravussin, Nat. Methods 9, 57 (2012)
M. J. Vosselman, W. D. van Marken Lichtenbelt, P. Schrauwen, Mol. Cell. Endocrinol. 379, 43 (2013)
J. E. Walker, Angew. Chem. Int. Ed. 37, 2308 (1998)
J. B. d. V. Weir, J. Physiol. 109, 1 (1949)
T. J. J. Zethof, J. A. M. Van Der Heyden, J. T. B. M. Tolboom, B. Olivier, Physiol. Behav. 55, 109 (1994)
Acknowledgments
This work was supported by the EPFL and funding was provided by the EU Ideas program (ERC-2012-AdG-320404). JA is the Nestlé Chair in Energy Metabolism and research in his laboratory was supported by institutional funds of the EPFL. We thank Norman Moullan for technical insight in cellular metabolism. We greatly thank Arnaud Bichat from EPFL Center of PhenoGenomics (CPG) for expert technical assistance in the surgical and behavioral experiments. We thank also the EPFL printed circuit board workshop (ACI) for assistance in sensors preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Padovani, R., Lehnert, T., Cettour-Rose, P. et al. Miniaturized implantable sensors for in vivo localized temperature measurements in mice during cold exposure. Biomed Microdevices 18, 1 (2016). https://doi.org/10.1007/s10544-015-0028-9
Published:
DOI: https://doi.org/10.1007/s10544-015-0028-9