Abstract
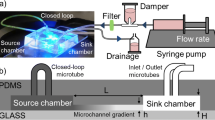
A biocompatible polydimethylsiloxane (PDMS) biomicrofluidic platform is designed, fabricated and tested to study protuberance growth of single plant cells in a micro-vitro environment. The design consists of an inlet to introduce the cell suspension into the chip, three outlets to conduct the medium or cells out of the chip, a main distribution chamber and eight microchannels connected to the main chamber to guide the growth of tip growing plant cells. The test cells used here were pollen grains which produce cylindrical protrusions called pollen tubes. The goal was to adjust the design of the microfluidic network with the aim to enhance the uniformly distributed positioning of pollen grains at the entrances of the microchannels and to provide identical fluid flow conditions for growing pollen tubes along each microchannel. Computational fluid analysis and experimental testing were carried out to estimate the trapping efficiencies of the different designs.









Similar content being viewed by others
References
C.G. Agudelo, A. Sanati Nezhad, M. Ghanbari, M. Packirisamy, A. Geitmann, J. Micromech. Microeng. 22, 115009 (2012)
C.G. Agudelo, A. Sanati Nezhad, M. Ghanbari, M. Naghavi, M. Packirisamy, R.B. Bhat, A. Geitmann, Plant J. 73, 1057–1068 (2013)
H. Andersson, A.V. Berg, Sensors Actuators 92, 315–325 (2003)
F. Bou Daher, A. Geitmann, Traffic 12, 1537–1551 (2012)
J. Bove, B. Vaillancourt, J. Kroeger, P.K. Hepler, P.W. Wiseman, A. Geitmann, Plant Physiol. 147, 1646–1658 (2008)
F. Bragheri, L. Ferrara, N. Bellini, K.C. Vishnubhatla, P. Minzioni, R. Ramponi, R. Osellame, I. Cristiani, J. Biophoton. 3, 234–243 (2010)
T. Braschler, R. Johann, M. Heule, L. Metref, P. Renaud, Lab Chip 5, 553–559 (2005)
S. Burgarella, S. Merlo, B. Dell'Anna, G. Zarola, M. Bianchessi, Microelectron. Eng. 87, 2124–2133 (2010)
Y. Chebli, A. Geitmann, Funct. Plant Sci. Biotechnol. 1, 232–245 (2007)
K.C. Cheung, P. Renaud, Solid State Electron. 50, 551–557 (2006)
J. Dinesh, in Proceedings of the COMSOL Conference, Bangalore, 2009
P. Fayant, O. Girlanda, Y. Chebli, C.E. Aubin, I. Villemure, A. Geitmann, Plant Cell Online 22, 2579–2593 (2010)
A. Geitmann, E. Parre, Sex Plant Reprod 17, 9–16 (2004)
I. Giouroudi, J. Kosel, C. Scheffer, Recent Patents Eng. 2, 114–121 (2008)
G. Grossmann, W.J. Guo, D.W. Ehrhardt, W.B. Frommer, R.V. Sit, S.R. Quake, M. Meier, Plant Cell Online 23, 4234–4240 (2011)
M.P. Hughes, H. Morgan, J. Phys. D. Appl. Phys. 31, 2205–2215 (1998)
R.S. Kanea, S. Takayamaa, E. Ostuni, D.E. Ingberb, G.M. Whitesides, Biomaterials 161, 1980–2004 (2007)
M. Kendrick, D. McIntyre, O. Ostroverkhova, JOSA B 26, 2189–2198 (2009)
S. Kobel, A. Valero, J. Latt, P. Renaud, M. Lutolf, Lab Chip 10, 857–863 (2010)
J. Kroeger, A. Geitmann, Plant Signal. Behav. 6, 1828–1858 (2011a)
J. Kroeger, A. Geitmann, Mech. Res. Commun. 42, 32–39 (2011b)
J.H. Kroeger, A. Geitmann, M. Grant, J. Theor. Biol. 253, 363–374 (2008)
J.H. Kroeger, F.B. Daher, M. Grant, A. Geitmann, Biophys. J. 97, 1822–1831 (2009)
S.W. Lee, J.Y. Kang, I.H. Lee, S.S. Ryu, S.M. Kwak, K.S. Shin, C. Kim, H.I. Jung, T.S. Kim, Sensors Actuators A Phys. 143, 64–69 (2008)
B. Ma, V. Ruwet, P. Corieri, R. Theunissen, M. Riethmuller, C. Darquenne, J. Aerosol Sci. 40, 403–414 (2009)
A. Melling, Meas. Sci. Technol. 8, 1406 (1997)
M. Mrksich, C.S. Chen, Y. Xia, L.E. Dike, D.E. Ingber, G.M. Whitesides, Proc. Natl. Acad. Sci. 93, 10775–10778 (1996)
E. Nuxoll, R. Siegel, Eng. Med. Biol. Mag. IEEE 28, 31–39 (2009)
A. Sanati Nezhad, M. Ghanbari, C.G. Agudelo, M. Packirisamy, R.B. Bhat, A. Geitmann, IEEE Sensors 13, 601–609 (2013a)
A. Sanati Nezhad, M. Packirisamy, R.B. Bhat, A. Geitmann, Proc. Natl. Acad. Sci. 110, 8093–8098 (2013b)
A. Sanati Nezhad, M. Packirisamy, R.B. Bhat, A. Geitmann, Lab Chip 13, 2599–2608 (2013c)
A. Sanati Nezhad, M. Packirisamy, R.B. Bhat, A. Geitmann, Biomed. Eng. IEEE Trans. (2013d). doi:10.1109/TBME.2013.2270914
J. Seo, C. Ionescu-Zanetti, J. Diamond, R. Lal, L.P. Lee, Appl. Phys. Lett. 84, 1973–1978 (2004)
S. Takayama, E. Ostuni, P. LeDuc, K. Naruse, D.E. Ingber, G.M. Whitesides, Nature 411, 1016 (2001)
W. Tan, T.A. Desai, Tissue Eng. 9, 255–267 (2003)
W.H. Tan, S. Takeuchi, Proc. Natl. Acad. Sci. 104, 1146–1151 (2007)
H. Vogler, C. Draeger, A. Weber, D. Felekis, C. Eichenberger, A.L. Routier-Kierzkowska, A. Boisson-Dernier, C. Ringli, B.J. Nelson, R.S. Smith, U. Grossniklaus, Plant J. 73, 617–627 (2012)
A.R. Wheeler, W.R. Throndset, R.J. Whelan, A.M. Leach, R.N. Zare, Y.H. Liao, K. Farrell, I.D. Manger, A. Daridon, Anal. Chem. 75, 3581–3586 (2003)
M. Yang, C.W. Li, J. Yang, Anal. Chem. 74, 3991–4001 (2002)
K. Yasuda, Sensors Actuators B Chem. 64, 128–135 (2000)
R. Zerzour, J. Kroeger, A. Geitmann, Dev. Biol. 334, 437–446 (2009)
M. Zhang, L. Wang, K. Xiao, W. Wen, Lab Chip 10, 1199–1203 (2010)
B. Ziaie, A. Baldi, M. Lei, Y. Gu, R.A. Siegel, Adv. Drug Deliv. Rev. 56, 145–172 (2004)
Acknowledgments
The authors acknowledge research support from the Fonds de recherche du Québec - Nature et Technologies (FQRNT) and Concordia Research Chair.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanati Nezhad, A., Ghanbari, M., Agudelo, C.G. et al. Optimization of flow assisted entrapment of pollen grains in a microfluidic platform for tip growth analysis. Biomed Microdevices 16, 23–33 (2014). https://doi.org/10.1007/s10544-013-9802-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-013-9802-8