Abstract
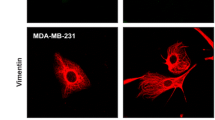
The paper reports the development of three dimensional (3-D) silicon microstructures and the utilization of these microenvironments for discriminating between normal fibroblast (HS68) and breast cancer cells (MDA-MB-231). These devices consist of arrays of microchambers connected with channels and were fabricated using a single-mask, single-isotropic-etch process. The behavior and response of normal fibroblast and breast cancer cells, two key cell types in human breast tumor microenvironments, were explored in terms of adhesion and growth in these artificial 3-D microenvironments having curved sidewalls. Breast cancer cells formed stable adhesions with the curved sidewalls however fibroblasts stretched and elongated their cytoskeleton and actin filaments inside the microchambers. Statistical analysis revealed that fibroblast cells grew on both flat silicon surfaces and inside the microchambers regardless of microchamber depth. However, the localization of breast cancer cells in these same substrates was dependent on the microchamber depth. After 72 h in culture, the ratio of the number of breast cancer cells on flat surfaces compared to breast cancer cells inside the microchambers was significantly decreased within the deeper microchambers; for microchambers having depths 88 μm less than 5% of the breast cancer cells grew on the flat surfaces. This behavior was sustained for 120 h, the longest time point examined. The results suggest that certain 3-D silicon microstructures have potential application as a tool to detect breast cancer cells and also as a platform for separating normal fibroblasts from breast cancer cells for cancer diagnosis applications.














Similar content being viewed by others
Notes
Trion
Trion
ALCATEL
ZEISS AXIOVERT 200
ZEISS LSM 510 META
ZEISS 1550
Physical Electronics Quanterra SXM
References
A. Benzeev, Biochim. Biophys. Acta 780, 197 (1984)
C.J. Bettinger, B. Orrick, A. Misra, R. Langer, J.T. Borenstein, Biomaterials 27, 2558 (2006). doi:10.1016/j.biomaterials.2005.11.029
N.A. Bhowmick, E.G. Neilson, H.L. Moses, Nature 432, 332 (2004). doi:10.1038/nature03096
J.L. Charest, A.J. Garcia, W.P. King, Biomaterials 28, 2202 (2007). doi:10.1016/j.biomaterials.2007.01.020
P. Clark, P. Connolly, A.S.G. Curtis, J.A.T. Dow, C.D.W. Wilkinson, J. Cell Sci. 99, 73 (1991)
M.J. Dalby, M.O. Riehle, S.J. Yarwood, C.D.W. Wilkinson, A.S.G. Curtis, Exp. Cell Res. 284, 274 (2003). doi:10.1016/S0014-4827(02)00053-8
E.T. denBraber, J.E. deRuijter, H.T.J. Smits, L.A. Ginsel, A.F. vonRecum, J.A. Jansen, Biomaterials 17, 1093 (1996). doi:10.1016/0142-9612(96)85910-2
D.E. Discher, P. Janmey, Y.L. Wang, Science 310, 1139 (2005). doi:10.1126/science.1116995
M.R. Dusseiller, D. Schlaepfer, M. Koch, R. Kroschewski, M. Textor, Biomaterials 26, 5917 (2005). doi:10.1016/j.biomaterials.2005.02.032
K. Gantz, L. Renaghan, and M. Agah, J. Micromechanics Microengineering 18 (2008).
H. Haga, S. Sasaki, K. Kawabata, E. Ito, T. Ushiki, T. Sambongi, Ultramicroscopy 82, 253 (2000). doi:10.1016/S0304-3991(99)00157-6
D.E. Ingber, J. Cell Sci. 104, 613 (1993)
R. Kalluri, M. Zeisberg, Nat. Rev. Cancer 6, 392 (2006). doi:10.1038/nrc1877
H.H. Liao, A.S. Andersson, D. Sutherland, S. Petronis, B. Kasemo, P. Thomsen, Biomaterials 24, 649 (2003). doi:10.1016/S0142-9612(02)00379-4
J.Y. Mai, C. Sun, S. Li, X. Zhang, Biomed. Microdevices 9, 523 (2007). doi:10.1007/s10544-007-9060-8
K. Matsuzaka, F. Walboomers, A. de Ruijter, J.A. Jansen, Clin. Oral Implants Res. 11, 325 (2000). doi:10.1034/j.1600-0501.2000.011004325.x
C. Oakley, D.M. Brunette, J. Cell Sci. 106, 343 (1993)
C. Oakley, N.A.F. Jaeger, D.M. Brunette, Exp. Cell Res. 234, 413 (1997). doi:10.1006/excr.1997.3625
T.H. Park, M.L. Shuler, Biotechnol. Prog. 19, 243 (2003). doi:10.1021/bp020143k
A.M. Rajnicek, S. Britland, C.D. McCaig, J. Cell Sci. 110, 2905 (1997)
K.M.K. Rao, H.J. Cohen, Mutat. Res. 256, 139 (1991)
Y.A. Rovensky, A.D. Bershadsky, E.I. Givargizov, L.N. Obolenskaya, J.M. Vasiliev, Exp. Cell Res. 197, 107 (1991). doi:10.1016/0014-4827(91)90486-E
T.P. Stossel, J. Cell Biol. 99, S15 (1984)
J.S. Strobl, M. Nikkhah, M. Agah, American Association for Cancer Research Special Conference on Advances in Breast Cancer Research: Genetics, Biology, and Clinical Applications, San Diego, CA, 2007
D. Tarin, C.B. Croft, J. Anat. 105, 189 (1969)
Y. Tseng, D. Wirtz, Biophys. J. 81, 1643 (2001)
A.M.P. Turner, N. Dowell, S.W.P. Turner, L. Kam, M. Isaacson, J.N. Turner, H.G. Craighead, W. Shain, J. Biomed. Mater. Res. 51, 430 (2000). doi:10.1002/1097-4636(20000905)51:3<430::AID-JBM18>3.0.CO;2-C
T.G. van Kooten, A.F. von Recum, Tissue Eng. 5, 223 (1999). doi:10.1089/ten.1999.5.223
X.F. Walboomers, H.J.E. Croes, L.A. Ginsel, J.A. Jansen, Biomaterials 19, 1861 (1998). doi:10.1016/S0142-9612(98)00093-3
X.F. Walboomers, H.J.E. Croes, L.A. Ginsel, J.A. Jansen, J. Biomed. Mater. Res. 47, 204 (1999a). doi:10.1002/(SICI)1097-4636(199911)47:2<204::AID-JBM10>3.0.CO;2-H
X.F. Walboomers, W. Monaghan, A.S.G. Curtis, J.A. Jansen, J. Biomed. Mater. Res. 46, 212 (1999b). doi:10.1002/(SICI)1097-4636(199908)46:2<212::AID-JBM10>3.0.CO;2-Y
G.M. Walker, H.C. Zeringue, D.J. Beebe, Lab Chip 4, 91 (2004). doi:10.1039/b311214d
R.A. Weinberg, The Biology of Cancer (Garland Science, Taylor & Francis Group, New York, 2007)
B. Wojciakstothard, A.S.G. Curtis, W. Monaghan, M. McGrath, I. Sommer, C.D.W. Wilkinson, Cell Motil. Cytoskelet. 31, 147 (1995). doi:10.1002/cm.970310207
C. Yao, Y. Lin, M.S. Chua, C.S. Ye, J. Bi, W. Li, Y.F. Zhu, S.M. Wang, Int. J. Cancer 121, 1949 (2007). doi:10.1002/ijc.22930
Acknowledgments
The authors would like to thank Micron Semiconductor Fabrication Facilities and Nanoscale Characterization and Fabrication Laboratory (NCFL) at Virginia Tech and Dr. Kristi DeCourcy at Fralin Biotechnology Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nikkhah, M., Strobl, J.S. & Agah, M. Attachment and response of human fibroblast and breast cancer cells to three dimensional silicon microstructures of different geometries. Biomed Microdevices 11, 429–441 (2009). https://doi.org/10.1007/s10544-008-9249-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-008-9249-5