Abstract
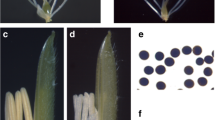
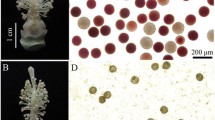
Male sterile line is a useful material for hybridization, but its sterility mechanism, especially proteomic profile, is still not entirely clear. In wheat (Triticum aestivum L.), a male sterile Bainong (BNS) genotype whose sterility could be manipulated by temperature was recently selected. We focused on the proteomic profile change of anthers from the male sterile line (SL) and its conversional line (CL). Two-dimensional gel electrophoresis and MALDI-TOF-MS technologies were utilized for proteomic profiles analysis. Differently abundant protein spots (over 2-fold, P < 0.05) were selected for identification analysis. Compared to CL, 24 up-regulated and 23 down-regulated proteins were identified in SL. Protein metabolism-related proteins, which included a ubiquitin-conjugating enzyme E2 (23 kDa) and an ATP-dependent Clp protease proteolytic subunit, were over-accumulated in SL anthers. Alcohol dehydrogenase ADH1A, fructose-bisphosphate aldolase, chloroplast fructose-bisphosphate aldolase, and NADP-dependent malic enzyme were notably down-regulated in SL anthers. Up-regulated prohibitin protein Wph and a translationallycontrolled tumor protein homolog, and down-regulated histone acetyltransferases HAT1 and HAT2, and DNA directed RNA polymerase subunit α were identified in SL anthers. These dramatically changed proteins may play a crucial role in abnormal anther development and pollen abortion in BNS.
Similar content being viewed by others
Abbreviations
- BNS:
-
Bainong male sterility
- CL:
-
conversional line
- GDH:
-
glutamate dehydrogenase
- GMS:
-
genic male sterility
- ROS:
-
reactive oxygen species
- SHMT:
-
serine hydroxymethyltransferase
- sHSPs:
-
small heat shock proteins
- SL:
-
sterile line
- TCTP:
-
translationally-controlled tumor protein homolog
References
Bewley, M. C., Graziano, V., Griffin, K., Flanagan, J. M.: Turned on for degradation: ATPase-independent degradation by ClpP. — J. struct. Biol. 165: 118–125, 2009.
Bindea, G., Mlecnik, B., Hackl, H., Charoentong, P., Tosolini, M., Kirilovsky, A., Fridman, W. H., Pages, F., Trajanoski, Z., Galon, J.: ClueGO: a cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. — Bioinformatics 25: 1091–1093, 2009.
Book, A.J., Smalle, J., Lee, K.H., Yang, P., Walker, J.M., Casper, S., Holmes, J.H., Russo, L.A., Buzzinotti, Z.W., Jenik, P.D., Vierstra, R.D.: The RPN5 subunit of the 26s proteasome is essential for gametogenesis, sporophyte development, and complex assembly in Arabidopsis. — Plant Cell 21: 460–478, 2009.
Brukhin, V., Gheyselinck, J., Gagliardini, V., Genschik, P., Grossniklaus, U.: The RPN1 subunit of the 26S proteasome in Arabidopsis is essential for embryogenesis. — Plant Cell 17: 2723–2737, 2005.
Chaudhury, A.M.: Nuclear genes controlling male fertility. — Plant Cell 5: 1277–1283, 1993.
Chen, L., Liu, Y.G.: Male sterility and fertility restoration in crops. — Annu. Rev. Plant Biol. 65: 579–606, 2013.
Chowdhury, I., Thompson, W.E., Thomas, K.: Prohibitins role in cellular survival through Ras-Raf-MEK-Erk pathway. — J. cell. Physiol. 229: 998–1004, 2013.
Clément, C., Audran, J.C.: Anther wall layers control pollen sugar nutrition inLilium. — Protoplasma 187: 172–181, 1995.
Clément, C., Burrus, M., Audran, J. C.: Floral organ growth and carbohydrate content during pollen development in Lilium. — Amer. J. Bot. 83: 459–469, 1996.
Conesa, A., Gotz, S., Garcia-Gomez, J.M., Terol, J., Talon, M., Robles, M.: Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. — Bioinformatics 21: 3674–3676, 2005.
Damerval, C., Vienne, D. D., Zivy, M., Thiellement, H.: Technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. — Electrophoresis 7: 52–54, 1986.
De Hoon, M.J., Imoto, S., Nolan, J., Miyano, S.: Open source clustering software. — Bioinformatics 20: 1453–1454, 2004.
Dewey, R.E., Timothy, D.H., Levings, C.S.: A mitochondrial protein associated with cytoplasmic male sterility in the T cytoplasm of maize. — Proc. nat. Acad. Sci. USA 84: 5374–5378, 1987.
Dolferus, R., Jacobs, M.: Polymorphism of alcohol dehydrogenase in Arabidopsis thaliana (L.) Heynh.: genetical and biochemical characterization. — Biochem. Genet. 22: 817–838, 1984.
Dorion, S., Lalonde, S., Saini, H. S.: Induction of male sterility in wheat by meiotic-stage water deficit is preceded by a decline in invertase activity and changes in carbohydrate metabolism in anthers. — Plant Physiol. 111: 137–145, 1996.
Ducos, E., Touzet, P., Boutry, M.: The male sterile G cytoplasm of wild beet displays modified mitochondrial respiratory complexes. — Plant J. 26: 171–180, 2001.
Eisen, M.B., Spellman, P.T., Brown, P.O., Botstein, D.: Cluster analysis and display of genome-wide expression patterns. — Proc. nat. Acad. Sci. USA 95: 14863–14868, 1998.
Feng, Y., Zhang, M., Guo, Q., Wang, G., Gong, J., Xu, Y., Wang, W.: Manipulation of monoubiquitin improves chilling tolerance in transgenic tobacco (Nicotiana tabacum). — Plant Physiol. Biochem. 75: 138–144, 2014.
Forde, B.G., Oliver, R.J., Leaver, C.J.: Variation in mitochondrial translation products associated with malesterile cytoplasms in maize. — Proc. nat. Acad. Sci. USA 75: 3841–3845, 1978.
Fritz, G., Kaina, B.: rhoB encoding a UV-inducible Rasrelated small GTP-binding protein is regulated by GTPases of the Rho family and independent of JNK, ERK, and p38 MAP kinase. — J. biol. Chem. 272: 30637–30644, 1997.
Ganesan, M., Han, Y.J., Bae, T.W., Hwang, O.J., Chandrasekhar, T., Shin, A.Y., Goh, C.H., Nishiguchi, S., Song, I.J., Lee, H.Y., Kim, J.I., Song, P.S.: Overexpression of phytochrome A and its hyperactive mutant improves shade tolerance and turf quality in creeping bentgrass and zoysiagrass. — Planta 236: 1135–1150, 2012.
Goetz, M., Godt, D. E., Guivarc'h, A., Kahmann, U., Chriqui, D., Roitsch, T.: Induction of male sterility in plants by metabolic engineering of the carbohydrate supply. — Proc. nat. Acad. Sci. USA 98: 6522–6527, 2001.
Guo, W., Zou, L.F., Li, Y.R., Cui, Y.P., Ji, Z.Y., Cai, L.L., Zou, H.S., Hutchins, W.C., Yang, C.H., Chen, G.Y.: Fructose-bisphophate aldolase exhibits functional roles between carbon metabolism and the hrp system in rice pathogen Xanthomonas oryzae pv. oryzicola. — PLoS One 7: e31855, 2012.
Hinojosa-Moya, J. J., Xoconostle-Cazares, B., Toscano-Morales, R., Arturo Ramirez-Ortega, F., Luis Cabrera-Ponce, J., Ruiz-Medrano, R.: Characterization of the pumpkin translationally-controlled tumor protein CmTCTP. — Plant Signal. Behav. 8: 1–8, 2013.
Ishikawa, T., Shigeoka, S.: Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. — Biosci. Biotechnol. Biochem. 72: 1143–1154, 2008.
Jamai, A., Salome, P.A., Schilling, S.H., Weber, A.P., McClung, C.R.: Arabidopsis photorespiratory serine hydroxymethyltransferase activity requires the mitochondrial accumulation of ferredoxin-dependent glutamate synthase. — Plant Cell. 21: 595–606, 2009.
Jumtee, K., Okazawa, A., Harada, K., Fukusaki, E., Takano, M., Kobayashi, A.: Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves. — J. Biosci. Bioeng. 108: 151–159, 2009.
Kanehisa, M., Goto, S.: KEGG: Kyoto encyclopedia of genes and genomes. — Nucl. Acids Res. 28: 27–30, 2000.
Kaul, M. L. H.: Male Sterility in Higher Plants. — Springer-Verlag, Berlin — Heidelberg 1988.
Konovalov, A.A., Ibragimova, S.S., Dorogova, N.V.: [Inheritance and expression of gametophyte gene Adh-P causing modification of alcohol dehydrogenase in pollen grains of the beet (Beta vulgaris L.)]. — Genetika 39: 1328–1337, 2003. [In Russ.]
Li, L., Ru, Z., Gao, Q., Jiang, H., Guo, F., Wu, S., SUN, Z.: Male sterility and thermo-photosensitivity characteristics of BNS in wheat. — Scientia agr. sin. 42: 3019–3027, 2009.
Liepman, A.H., Olsen, L.J.: Peroxisomal alanine: glyoxylate aminotransferase (AGT1) is a photorespiratory enzyme with multiple substrates in Arabidopsis thaliana. — Plant J. 25: 487–498, 2001.
Link, A.J., LaBaer, J.: Trichloroacetic acid (TCA) precipitation of proteins. — Cold Spring Harbor Protoc. 2011: 993–994, 2011.
Livak, K.J., Schmittgen, T.D.: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. — Methods 25: 402–408, 2001.
Mariani, C., Beuckeleer, M.D., Truettner, J., Leemans, J., Goldberg, R.B.: Induction of male sterility in plants by a chimaeric ribonuclease gene. — Nature 347: 737–741, 1990.
Mariani, C., Goldberg, R.B., Leemans, J.: Engineered male sterility in plants. — Symp. Soc. exp. Biol. 45: 271–279, 1991.
McCarthy, F.M., Wang, N., Magee, G.B., Nanduri, B., Lawrence, M.L., Camon, E.B., Barrell, D.G., Hill, D.P., Dolan, M.E., Williams, W.P., Luthe, D.S., Bridges, S.M., Burgess, S.C.: AgBase: a functional genomics resource for agriculture. — BMC Genom. 7: 229, 2006.
Millar, A.A., Clemens, S., Zachgo, S., Giblin, E.M., Taylor, D.C., Kunst, L.: CUT1, an Arabidopsis gene required for cuticular wax biosynthesis and pollen fertility, encodes a very-long-chain fatty acid condensing enzyme. — Plant Cell 11: 825–838, 1999.
Mishra, S., Ande, S.R., Nyomba, B.L.: The role of prohibitin in cell signaling. — FEBS J. 277: 3937–3946, 2010.
Moreno, J.I., Martin, R., Castresana, C.: Arabidopsis SHMT1, a serine hydroxymethyltransferase that functions in the photorespiratory pathway influences resistance to biotic and abiotic stress. — Plant J. 41: 451–463, 2005.
Perkins, D.N., Pappin, D.J., Creasy, D.M., Cottrell, J.S.: Probability-based protein identification by searching sequence databases using mass spectrometry data. — Electrophoresis 20: 3551–3567, 1999.
Popov, V.N., Eprintsev, A.T., Fedorin, D.N., Igamberdiev, A.U.: Succinate dehydrogenase in Arabidopsis thaliana is regulated by light via phytochrome A. — FEBS Lett. 584: 199–202, 2010.
Raines, C.A.: Transgenic approaches to manipulate the environmental responses of the C3 carbon fixation cycle. — Plant Cell Environ. 29: 331–339, 2006.
Reid, B.G., Fenton, W.A., Horwich, A.L., Weber-Ban, E.U.: ClpA mediates directional translocation of substrate proteins into the ClpP protease. — Proc. nat. Acad. Sci. USA 98: 3768–3772, 2001.
Ricoult, C., Echeverria, L.O., Cliquet, J.B., Limami, A.M.: Characterization of alanine aminotransferase (AlaAT) multigene family and hypoxic response in young seedlings of the model legume Medicago truncatula. — J. exp. Bot. 57: 3079–3089, 2006.
Shannon, P., Markiel, A., Ozier, O., Baliga, N.S., Wang, J.T., Ramage, D., Amin, N., Schwikowski, B., Ideker, T.: Cytoscape: a software environment for integrated models of biomolecular interaction networks. — Genome Res. 13: 2498–2504, 2003.
Simpson, R. J.: Proteins and Proteomics: A Laboratory Manual. — Cold Spring Harbor Laboratory Press, Cold Spring Harbor 2003.
Sjogren, L.L., Stanne, T.M., Zheng, B., Sutinen, S., Clarke, A.K.: Structural and functional insights into the chloroplast ATP-dependent Clp protease in Arabidopsis. — Plant Cell 18: 2635–2649, 2006.
Smalle, J., Kurepa, J., Yang, P., Babiychuk, E., Kushnir, S., Durski, A., Vierstra, R.D.: Cytokinin growth responses in Arabidopsis involve the 26S proteasome subunit RPN12. — Plant Cell 14: 17–32, 2002.
Smalle, J., Kurepa, J., Yang, P., Emborg, T.J., Babiychuk, E., Kushnir, S., Vierstra, R.D.: The pleiotropic role of the 26S proteasome subunit RPN10 in Arabidopsis growth and development supports a substrate-specific function in abscisic acid signaling. — Plant Cell 15: 965–980, 2003.
Somerville, C.R., Ogren, W.L.: Photorespiration-deficient mutants of Arabidopsis thaliana lacking mitochondrial serine transhydroxymethylase activity. — Plant Physiol. 67: 666–671, 1981.
Song, J., Hedgcoth, C.: A chimeric gene (orf256) is expressed as protein only in cytoplasmic male-sterile lines of wheat. — Plant mol. Biol. 26: 535–539, 1994.
Su, Q.Z., Cheng, W., Li, Y.: Morphological and structural observations on anther and microspore developments of male sterile line BNS in wheat. — J. Henan Inst. Sci. Technol. 39: 5–9, 2011.
Ueda, M., Matsui, K., Ishiguro, S., Sano, R., Wada, T., Paponov, I., Palme, K., Okada, K.: The HALTED ROOT gene encoding the 26S proteasome subunit RPT2a is essential for the maintenance of Arabidopsis meristems. — Development 131: 2101–2111, 2004.
Uematsu, K., Suzuki, N., Iwamae, T., Inui, M., Yukawa, H.: Increased fructose 1,6-bisphosphate aldolase in plastids enhances growth and photosynthesis of tobacco plants. — J. exp. Bot. 63: 3001–3009, 2012.
Vierstra, R.D.: The ubiquitin-26S proteasome system at the nexus of plant biology. — Natur. Rev. mol. Cell Biol. 10: 385–397, 2009.
Voges, D., Zwickl, P., Baumeister, W.: The 26S proteasome: a molecular machine designed for controlled proteolysis. — Annu. Rev. Biochem. 68: 1015–1068, 1999.
Wang, A., Xia, Q., Xie, W., Datla, R., Selvaraj, G.: The classical Ubisch bodies carry a sporophytically produced structural protein (RAFTIN) that is essential for pollen development. — Proc. nat. Acad. Sci. USA 100: 14487–14492, 2003.
Wang, K., Gao, F., Ji, Y., Liu, Y., Dan, Z., Yang, P., Zhu, Y., Li, S.: ORFH79 impairs mitochondrial function via interaction with a subunit of electron transport chain complex III in Honglian cytoplasmic male sterile rice. — New Phytol. 198: 408–418, 2013.
Waters, E.R.: The evolution, function, structure, and expression of the plant sHSPs. — J. exp. Bot. 64: 391–403, 2013.
Weber-Ban, E.U., Reid, B.G., Miranker, A.D., Horwich, A.L.: Global unfolding of a substrate protein by the Hsp100 chaperone ClpA. — Nature 401: 90–93, 1999.
Zhou, M., Ru, Z., Luo, Y., Luo, P., Li, Q., Guo, X., Zhou, P.: Male fertility transformation of two-line wheat sterile lines BNS. — J. nucl. agr. Sci. 24: 887–894, 2010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgments: We are grateful for grants from the National Basic Research Program (973) of China (No. 2007CB109006) and the Foundation & Advanced Technology Research Program of Henan Province (Nos. 102300410127 and 122300410011).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, Y.Y., Li, Y., Fu, Q.G. et al. Anther proteomic characterization in temperature sensitive Bainong male sterile wheat. Biol Plant 59, 273–282 (2015). https://doi.org/10.1007/s10535-015-0486-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10535-015-0486-1