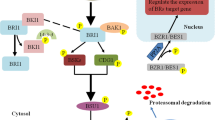
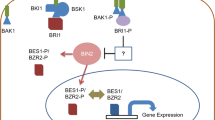
Abstract
Brassinosteroids (BRs) are polyhydroxylated steroidal plant hormones that play pivotal role in the regulation of various plant growth and development processes. BR biosynthetic or signaling mutants clearly indicate that these plant steroids are essential for regulating a variety of physiological processes including cellular expansion and proliferation, vascular differentiation, male fertility, timing senescence, and leaf development. Moreover, BRs regulate the expression of hundreds of genes, affect the activity of numerous metabolic pathways, and help to control overall developmental programs leading to morphogenesis. On the other hand, the potential application of BRs in agriculture to improve growth and yield under various stress conditions including drought, salinity, extreme temperatures, and heavy metal (Cd, Cu, Al, and Ni) toxicity, is of immense significance as these stresses severely hamper the normal metabolism of plants. Keeping in mind the multifaceted role of BRs, an attempt has been made to cover the various aspects mediated by BRs particularly under stress conditions and a possible mechanism of action of BRs has also been suggested.
Similar content being viewed by others
Abbreviations
- 28norCS:
-
28-norcasterone
- 6deoxoCS:
-
6-deoxocasterone
- 6-deoxoTY:
-
6-deoxotyphasterol
- BL:
-
brassinolide
- BRI1:
-
brassinosteroids insensitive I
- BRs:
-
brassinosteroids
- CAT:
-
catalase
- CS:
-
castasterone
- EBL:
-
24-epibrassinolide
- HBL:
-
28-homobrassinolide
- LRR:
-
leucine rich repeat
- MT-sHSP:
-
mitochondrial small heat shock protein
- NPR1:
-
non-expressor of pathogenesis related genes 1
- P5C:
-
pyrroline-5-carboxylase
- POX:
-
peroxidase
- PR-1:
-
pathogenesis related 1
- PS II:
-
photosystem II
- ROS:
-
reactive oxygen species
- S/T kinase:
-
serine/threonine kinase
- SOD:
-
superoxide dismutase
- TE:
-
teasterone
- TY:
-
typhasterol
References
Abdullahi, B.A., Gu, X., Gan, Q., Yang, Y.: Brassinolide amelioration of aluminium toxicity in mung bean seedling growth. — J. Plant. Nutr. 26: 1725–1734, 2003.
Ahammed, G.J., Zhou, Y.H., Xia, X.J., Mao, W.H., Shi, K., Yu, J.Q.: Brassinosteroid regulates secondary metabolism in tomato towards enhanced tolerance to phenanthrene. — Biol. Plant. 57: 154–158, 2013.
Alam, M.M., Hayat, S., Ali, B., Ahmad, A.: Effect of 28-homobrassinolide on nickel induced changes in Brassica juncea. — Photosynthetica 45: 139–142, 2007.
Ali, B., Hasan, S.A., Hayat, S., Hayat, Q., Yadav, S., Fariduddin, Q., Ahmad, A.: A role of brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L.) Wilczek. — Environ. exp. Bot. 62: 153–159, 2008a.
Ali, B., Hayat, S., Ahmad, A.: 28-homobrassinolide ameliorates the saline stress in Cicer arietinum L. — Environ. exp. Bot. 59: 217–223, 2007.
Ali, Q., Athar, H.R., Ashraf, M.: Modulation of growth, photosynthetic capacity and water relations in salt stressed wheat plants by exogenously applied 24-epibrassinolide. — Plant. Growth. Regul. 56: 107–116, 2008b.
Allen, D.J., Ort, D.R.: Impact of chilling temperatures on photosynthesis in warm-climate plants. — Trends Plant. Sci. 6: 36–42, 2001.
Anuradha, S., Rao, S.S.R.: Effect of brassinosteroids on radish (Raphanus sativus L.) seedlings growing under cadmium stress. — Plant Soil Environ. 53: 465–472, 2007a.
Ashraf, M., Akram, N.A., Arteca, R.N., Foolad, M.R.: The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. — Crit. Rev. Plant. Sci. 29: 162–190, 2010.
Ashraf, M., Foolad, M.R.: Roles of glycinebetaine and proline in improving plant abiotic stress resistance. — Environ. exp. Bot. 59: 206–216, 2007.
Bajguz, A., Hayat, S.: Effects of brassinosteroids on the plant responses to environmental stresses. — Plant. Physiol. Biochem. 47: 1–8, 2009.
Berry, J., Björkman, O.: Photosynthetic response and adaptation to temperature in higher plants. — Annu. Rev. Plant Physiol. 31: 491–543, 1980.
Caño-Delgado, A., Yin, Y., Yu, C., Vafeados, D., Mora-García, S., Cheng, J.C., Nam, K.H., Li, J., Chory, J.: BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. — Development 131: 5341–5351, 2004.
Cao, S., Xu, Q., Cao, Y., Qian, K., An, K., Zhu, Y., Binzeng, H., Zhao, H., Kuai, B.: Loss-of-function mutations in DET2 gene lead to an enhanced resistance to oxidative stress in Arabidopsis. — Physiol. Plant. 123: 57–66, 2005.
Catterou, M., Dubois, F., Schaller, H., Aubanelle, L., Vilcot, B., Sangwan, N.B.S., Sangwan, R.S.: Brassinosteroids, microtubules and cell elongation in Arabidopsis thaliana. I. Molecular, cellular and physiological characterization of the Arabidopsis bul1 mutant, defective in the Δ(7)-sterol-C5-desaturation step leading to brassinosteroids biosynthesis. — Planta 212: 659–672, 2001.
Choe, S.: Brassinosteroid biosynthesis and inactivation. — Physiol. Plant. 126: 539–548, 2006.
Choudhary, S.P., Yu, J.Q., Yamaguchi-Shinozaki, K., Shinozaki, K., Lam-Son, P.T.: Benefits of brassinosteroid crosstalk. — Trends Plant Sci. 17: 594–605, 2012.
Clouse, S.D., Sasse, J.M.: Brassinosteroids: essential regulators of plant growth and development. — Annu. Rev. Plant Physiol. Plant mol. Biol. 49: 427–451, 1998.
Divi, U.K., Rahman, T., Krishna, P.: Brassinosteroids-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. — BMC Plant Biol. 10: 151, 2010.
Ding, H.D., Zhu, X.H., Zhu, Z.W., Yang, S.J., Zha, D.S., Wu, X.X.: Amelioration of salt-induced oxidative stress in eggplant by application of 24-epibrassinolide. — Biol. Plant. 56: 767–770, 2012
Fariduddin, Q., Khanam, S., Hasan, S.A., Ali, B., Hayat, S., Ahmad, A.: Effect of 28-homobrassinolide on drought stress induced changes in photosynthesis and antioxidant system of Brassica juncea L. — Acta Physiol. Plant. 31: 889–897, 2009a.
Fariduddin, Q., Yusuf, M., Chalkoo, S., Hayat, S., Ahmad, A.: 28-homobrassinolide improves growth and photosynthesis in Cucumis sativus L. through an enhanced antioxidant system in the presence of chilling stress. — Photosynthetica 49: 55–64, 2011.
Fariduddin, Q., Yusuf, M., Hayat, S., Ahmad, A.: Effect of 28-homobrassinolide on antioxidant capacity and photosynthesis in Brassica juncea plants exposed to different levels of copper. — Environ. exp. Bot. 66: 418–424, 2009b.
Friedrichsen, D.M., Joazeiro, C.A.P., Li, J., Hunter, T., Chory, J.: Brassinosteroid-Insensitive-I is a ubiquitously expressed leucine-rich repeat receptor serine/threonine kinase. — Plant Physiol. 123: 1247–1255, 2000.
Gille, G., Sigler, K.: Oxidative stress in living cells. — Folia microbiol. 2: 131–152, 1995.
Goda, H., Shimada, Y., Asami, T., Fujioka, S., Yoshida, S.: Microarray analysis of brassinosteroid-regulated genes in Arabidopsis. — Plant Physiol. 130: 1319–1334, 2002.
Gomes, M.M.A.: Physiological effects related to brassinosteroid application in plants. — In: Hayat, S., Ahmad, A. (ed.): Brassinosteroids: a Class of Plant Hormones. Pp. 193–--. Springer, Dordrecht — Heidelberg — London — New York 2011.
Gratao, P.L, Gomes-Junior, R.A., Delite, F.S., Lea, P.J., Azevedo, R.A.: Antioxidants stress responses of plants to cadmium. — In: Khan, N.A., Samiullah (ed.): Cadmium Toxicity and Tolerance in Plants. Pp. 1–34. Alpha Science International, Oxford 2006.
Hasan, S.A., Hayat, S., Ali, B., Ahmad, A.: 28-homobrassinolide protects chickpea (Cicer arietinum) from cadmium toxicity by stimulating antioxidant. — Environ. Pollut. 151: 60–66, 2008.
Hayat, S., Ali, B., Hasan, S.A., Ahmad, A.: Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. — Environ. exp. Bot. 60: 33–41, 2007.
Hayat, S., Hasan, S.A., Yusuf, M., Hayat, Q., Ahmad, A.: Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. — Environ. exp. Bot. 69: 105–112, 2010.
Hopkins, W.J.: Physiology of plants under stress. — In: Hopkins, W.J. (ed.): Introduction to Plant Physiology. 3rd Ed. Pp. 459–479. John Wiley & Sons, New York 1995.
Huang, L.F., Zheng, J.H., Zhang, Y.Y., Hu, W.H., Mao, W.H., Zhou, Y.H., Yu, J.Q.: Diurnal variations in gas exchange, chlorophyll fluorescence quenching and light allocation in soybean leaves: the cause for midday depression in CO2 assimilation. — Sci. Hort. 110: 214–218, 2006.
Jaleel, C.A., Manivannan, P., Lakshmanan, G.M.A., Gomathinayagam, M., Panneerselvam, R.: Alterations in morphological parameters and photosynthetic pigment responses of Catharanthus roseus under soil water deficits. — Colloids Surf. B: Biointerfaces 61: 298–303, 2008.
Janeczko, A., Gullner, G., Skoczowski, A., Dubert, F., Barna, B.: Effects of brassinosteroid infiltration prior to cold treatment on ion leakage and pigment contents in rape leaves. — Biol. Plant. 51: 355–358, 2007.
Janeckzo, A., Koscielniak, J., Pilipowicz, M., Szarek-Lukaszewska, G., Skoczowski, A.: Protection of winter rape photosystem 2 by 24-epibrassinolide under cadmium stress. — Photosynthetica 43: 293–298, 2005.
Jiang, Y.P., Cheng, F., Zhou, Y.H., Xia, X.J., Mao, W.H., Shi, K., Chen, Z., Yu, J.Q.: Cellular glutathione redox homeostasis plays an important role in the brassinosteroidinduced increase in CO2 assimilation in Cucumis sativus. — New Phytol. 194: 932–943, 2012.
Kagale, S., Divi, U.K., Krochko, J.E., Keller, W.A., Krishna, P.: Brassinosteroids confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. — Planta 225: 353–364, 2007.
Kim, T.W., Wang, Z.Y.: Brassinosteroid signal transduction from receptor kinases to transcription factors. — Annu. Rev. Plant Physiol. Plant mol. Biol. 61: 681–704, 2010.
Kinoshita, K., Cano-Delgado, A., Seto, H., Hiranuma, S., Fujioka, S., Yoshida, S., Chory, J.: Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. — Nature 433: 167–171, 2005.
Li, Y.H., Liu, Y.J., Xu, X.L., Jin, M., An, L.Z., Zhang, H.: Effect of 24-epibrassinolide on drought stress-induced changes in Chorispora bungeana. — Biol. Plant. 56: 192–196, 2012
Lin, Y.C., Kao, C.H.: Proline accumulation induced by excess nickel in detached rice leaves. — Biol. Plant. 51: 351–354, 2007.
Mandava, N.B.: Plant growth-promoting brassinosteroids. — Annu. Rev. Plant Physiol. Plant mol. Biol. 39: 23–52, 1988.
Mazorra, L.M., Nunez, M., Hechavarria, M., Coll, F., Sanchez-Blanco, M.J.: Influence of brassinosteroids on antioxidant enzymes activity in tomato under different temperatures. — Biol. Plant. 45: 593–596, 2002.
Mitchell, J.W., Mandhava, N.B., Worley, J.F., Plimmer, J.R., Smith, M.V.: Brassins — a new family of plant hormones from rape pollen. — Nature 255: 1065–1066, 1970.
Mittler, R., Vanderauwera, S., Suzuki, N., Miller, G., Tognetti, V.B., Vandepoele, K., Gollery, M., Shulaev, V., Van Breusegem, F.: ROS signaling: the new wave? — Trends Plant Sci. 16: 300–309, 2011.
Mussig, C., Fischer, S., Altamann, T.: Brassinosteroid-regulated gene expression. — Plant Physiol. 129: 1241–1251, 2002.
Navrot, N., Rouhier, N., Gelhaye, E., Jacquot, J.: Reactive oxygen species generation and antioxidant systems in plant mitochondria. — Physiol. Plant. 129: 185–195, 2007.
Nemhauser, Jennifer, L., Mockler, T.C., Chory, J.: Interdependency of brassinosteroid and auxin signaling in Arabidopsis. — PLoS Biol. 2: E258, 2004.
Nunez, M., Mazzafera, P., Mazorra, L.M., Siqueira, W.J., Zullo, M.A.T.: Influence of a brassinsteroid analogue on antioxidant enzymes in rice grown in culture medium with NaCl. — Biol Plant. 47: 67–70, 2003.
Ogweno, J.O., Song, X.S., Shi, K., Hu, W.H., Mao, W.H., Zhou, Y.H., Yu, J.Q., Nogues, S.: Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. — J. Plant. Growth. Regul. 27: 49–57, 2008.
Özdemir, F., Bor, M., Demiral, T., Turkan, I.: Effects of 24-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa L.) under salinity stress. — Plant. Growth Regul. 42: 203–211, 2004.
Prasad, M.N.V.: Heavy Metal Stress in Plants — from Biomolecules to Ecosystems. — Springer-Verlag, Berlin — Heidelberg. 2004.
Ruley, A.T., Sharma, N.C., Sahi, S.V.: Antioxidant defense in a lead accumulating plant, Sesbania drummondii. — Plant Physiol. Biochem. 42: 899–906, 2004.
Salveit, M.E.: Chilling injury is reduced in cucumber and rice seedlings in tomato pericarp discs by heat-shocks applied after chilling. — Post. Harvest. Biol. Technol. 21: 169–177, 2001.
Sasse, J.M.: Physiological actions of brassinosteroids: an update. — Plant. Growth Regul. 22: 276–288, 2003.
Sharma, P., Bhardwaj, R., Arora, N., Arora, H.K., Kumar, A.: Effects of 28-homobrassinolide on nickel uptake, protein content and antioxidative defence system in Brassica juncea. — Biol. Plant. 52: 767–770, 2008.
Sharma, P., Bhardwaj, R.: Effects of 24-epibrassinolide on growth and metal uptake Brassica juncea L. under copper metal stress. — Acta Physiol. Plant. 29: 259–263, 2007.
Sharma, P., Dubey, R.S.: Modulation of nitrate reductase activity in rice seedlings under aluminium toxicity and water stress: role of osmolytes as enzyme protectant. — J. Plant. Physiol. 162: 854–864, 2005.
Simoes-Araujo, J.L., Rumjanek, N.G., Margis-Pinheiro, M.: Small heat shock proteins genes are differentially expressed in distinct varieties of common bean. — Braz. J. Plant. Physiol. 15: 33–41, 2003.
Simonovicova, M., Tamas, L., Huttova, J., Mistrik. I.: Effect of aluminum on oxidative stress related enzymes activities in barley roots. — Biol Plant. 48: 261–266, 2004.
Singh, I., Shono, M.: Physiological and molecular effects of 24-epibrassinolide, a brassinosteroid on thermotolerance of tomato. — Plant. Growth Regul. 47: 111–119, 2005.
Steffens, G.L.: US department of agriculture Brassins project: 1970–1980. — In: Cutler, H.G., Yokota, T., Adam, G. (ed.): Brassinosteroids: Chemistry, Bioactivity, and Applications. Pp. 2–17. American Chemical Society, Washington 1991.
Taiz, L., Zeiger, E.: Plant Physiology. Pp. 607–611. Sinauer Associates, Sunderland 2004.
Teale, W.D., Ditengou, F.A., Dovzhenko, A.D., Li, X., Molendijk, A.M., Ruperti, B., Paponov, I., Palme, K.: Auxin as a model for the integration of hormonal signal processing and transduction. — Mol. Plant 1: 229–237, 2008.
Turkan, I., Demiral, T.: Recent developments in understanding salinity tolerance. — Environ. exp. Bot. 67: 2–9, 2009.
Upreti, K.K., Murti, G.S.R.: Effects of brassinosteroids on growth, nodulation, phytohormone content and nitrogenase activity in French bean under water stress. — Biol. Plant. 48: 407–411, 2004.
Vardhini, B.V., Rao, S.S.R.: Amelioration of osmotic stress by brassinosteroids on seed germination and seedling growth of three varieties of sorghum. — Plant. Growth Regul. 41: 25–31, 2003.
Vassilev, A., Yordanov, I.: Reductive analysis of factors limiting growth of cadmium-treated plants: a review. — Bulg. J. Plant Physiol. 23: 114–133, 1997.
Wang, Z.Y., Wang, Q., Chong, K., Wang, F., Wang, L., Bai, M., Jia, C.: The brassinosteroid signal transduction pathway. — Cell. Res. 16: 427–434, 2006.
Xia, X.J., Huang, L.-F., Zhou, Y.-H., Mao, W.-H., Shi, K., Wu, J.-X., Asami, T., Chen, Z., Yu, J.-Q.: Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. — Planta 230: 1185–1196, 2009.
Yusuf, M., Fariduddin, Q., Ahmad, A.: 24-Epibrassinolide modulates growth, nodulation, antioxidant system, and osmolyte in tolerant and sensitive varieties of Vigna radiata under different levels of nickel: a shotgun approach. — Plant Physiol. Biochem. 57: 143–153, 2012.
Yusuf, M.: Effect of brassinosteroids on nickel induced changes in Vigna radiata. — PhD. Thesis, Aligarh Muslim University, Aligarh 2011.
Yusuf, M., Fariduddin, Q., Hayat, S., Hasan, S.A., Ahmad, A.: Protective responses of 28-homobrssinolide in cultivars of Triticum aestivum with different levels of nickel. — Arch. Environ. Contam. Toxicol. 60: 68–76, 2011.
Zhang, J.H., Huang, W.D., Liu, Y.P., Pan, Q.H.: Effects of temperature acclimation pre-treatment on the ultrastructure of mesophyll cells in young grape plants (Vitis vinifera L. cv. Jingxiu) under cross-temperature stresses. — J. Integ. Plant. Biol. 47: 959–970, 2005.
Zhang, M., Zhai, Z., Tian, X., Duan, L., Li, Z.: Brassinolide alleviated the adverse effect of water deficits on photosynthesis and the antioxidant of soybean (Glycine max L.). — Plant Growth Regul. 56: 257–264, 2008.
Zurek, D.M., Rayle, D.L., McMorris, T.C., Clouse, S.D.: Investigation of gene expression, growth kinetics, and wall extensibility during brassinosteroid regulated stem elongation. — Plant Physiol. 104: 503–513, 1994.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements: M. Yusuf gratefully acknowledges the financial assistance rendered by the University Grant Commission, New Delhi, India in a form of the Dr. D.S. Kothari Postdoctoral Fellowship [F.4-2/2006(BSR) 13-608/2012/BSR].
Rights and permissions
About this article
Cite this article
Fariduddin, Q., Yusuf, M., Ahmad, I. et al. Brassinosteroids and their role in response of plants to abiotic stresses. Biol Plant 58, 9–17 (2014). https://doi.org/10.1007/s10535-013-0374-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10535-013-0374-5