Abstract
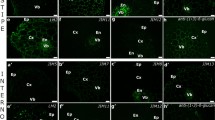
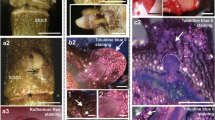
The aim of the present study was to describe the occurrence of three pectic epitopes, recognized by JIM7, LM19, and LM5 antibodies, during somatic (SE) and zygotic (ZE) embryogenesis in Arabidopsis thaliana. The epitopes recognized by JIM7 and LM19 antibodies showed different distributions during SE stages. Moreover, in the early stages of somatic embryo development, a cytoplasmic occurrence of LM19 epitope was detected. Distribution of a pectic epitope recognized by LM5 antibody corresponded to a vascular system differentiation pattern. Occurrence of LM5 epitope was the same in both zygotic and somatic embryos and often restricted to newly synthesized walls of two adjacent cells. These data suggest that both low and high methyl-esterified pectins (recognized by LM19 and JIM7 antibodies, respectively) are developmentally regulated during SE stages and (1→4)-β-D-galactan epitope (recognized by LM5 antibody) may play a role in cell cytokinesis.
Similar content being viewed by others
Abbreviations
- HG:
-
homogalacturonan
- IZE:
-
immature zygotic embryo
- MS:
-
Murashige and Skoog
- PEGs:
-
cuticular pegs
- RG:
-
rhamnogalacturonan
- SAM:
-
shoot apical meristem
- SE:
-
somatic embryogenesis
- ZE:
-
zygotic embryogenesis
References
Baluska, F., Samaj, J., Wojtaszek, P., Volkmann, D., Menzel, D.: Cytoskeleton-plasma membrane-cell wall continuum in plants. Emerging links revisited. — Plant Physiol. 133: 482–491, 2003.
Bobak, M., Samaj, J., Hlinkova, E., Hlavacka, A., Ovecka, M.: Extracellular matrix in early stages of direct somatic embryogenesis in leaves of Drosera spathulata. — Biol. Plant. 47: 161–166, 2003/4.
Bouton, S., Leboeuf, E., Mouille, G., Leydecker, M.-T., Talbotec, J., Granier, F., Lahaye, M., Höfte, H., Truong, H.-N.: QUASIMODO1 encodes a putative membrane-bound glycosyltransferase required for normal pectin synthesis and cell adhesion in Arabidopsis. — Plant Cell 14: 2577–2590, 2002.
Brownlee, C.: Role of the extracellular matrix in cell-cell signalling: paracrine paradigms. — Curr. Opin. Plant Biol. 5: 396–401, 2002.
Bush, M.S., McCann, M.C.: Pectic epitopes are differentially distributed in the cell walls of potato (Solanum tuberosum) tubers. — Physiol. Plant. 107: 201–213, 1999.
Bush, M.S., Marry, M., Huxham, M.I., Jarvis, M.C., McCann, M.C.: Developmental regulation of pectic epitopes during potato tuberisation. — Planta 213: 869–880, 2001.
Chapman, A., Blervacq, A.-S., Hendriks, T., Slomianny, C., Vasseur, J., Hilbert, J.-L.: Cell wall differentiation during early somatic embryogenesis in plants. II. Ultrastructural study and pectin immunolocalization on chicory embryos. — Can. J. Bot. 78: 824–831, 2000.
Chen, W., Stoddard, F.L., Baldwin, T.C.: Developmental regulation of mannan, arabinogalactan-protein, and pectic epitopes in pistils of Vicia faba (faba bean). — Int. J. Plant Sci. 167: 919–932, 2006.
Dobrowolska, I., Majchrzak, O., Baldwin, T.C., Kurczynska, E.U.: Differences in protodermal cell wall structure in zygotic and somatic embryos of Daucus carota (L.) cultured on solid and in liquid media. — Protoplasma 249: 117–129, 2012.
Elviana, M., Rohani, E.R., Ismanizan, I., Normah, M.N.: Morphological and histological changes during the somatic embryogenesis of mangosteen. — Biol. Plant. 55: 731–736, 2011.
Ermel, F.F., Follet-Gueye, M.-L., Cibert, C., Vian, B., Morvan, C., Catesson, A.-M., Goldberg, R.: Differential localization of arabinan and galactan side chains of rhamnogalacturonan 1 in cambial derivatives. — Planta 210: 732–740, 2000.
Femenia, A., Garosi, P., Roberts, K., Waldron, K.W., Selvendran, R.R., Robertson, J.A.: Tissue-related changes in methyl-esterification of pectic polysaccharides in cauliflower (Brassica oleracea L. var. botrytis) stems. — Planta 205: 438–444, 1998.
Femenia, A., Waldron, K.W., Robertson, J.A., Selvendran, R.R.: Compositional and structural modification of the cell wall of cauliflower (Brassica oleracea L. var botrytis) during tissue development and plant maturation. — Carbohydr. Polymer. 39: 101–108, 1999.
Freshour, G., Clay, R.P., Fuller, M.S., Albersheim, P., Darvill, A.G., Hahn, M.G.: Developmental and tissue-specific structural alterations of the cell-wall polysaccharides of Arabidopsis thaliana roots. — Plant Physiol. 110: 1413–1429, 1996.
Gaj, M.D.: Direct somatic embryogenesis as a rapid and efficient system for in vitro regeneration of Arabidopsis thaliana. — Plant Cell Tissue Organ Cult. 64: 39–46, 2001.
Gamborg, O.L., Miller, R.A., Ojima, K.: Nutrient requirements of suspension cultures of soybean root cells. — Exp. Cell Res. 50: 151–158, 1968.
Ikeda-Iwai, M., Satoh, S., Kamada, H.: Establishment of a reproducible tissue culture system for the induction of Arabidopsis somatic embryos. — J. exp. Bot. 53: 1575–1580, 2002.
Iwai, H., Kikuchi, A., Kobayashi, T., Kamada, H., Satoh, S.: High levels of non-methylesterified pectins and low levels of peripherally located pectins in loosely attached nonembryogenic callus of carrot. — Plant Cell Rep. 18: 561–566, 1999.
Jarvis, M.C.: Structure and properties of pectin gels in plant cell walls. — Plant Cell Environ. 7: 153–164, 1984.
Jarvis, M.C., Briggs, S.P.H., Knox, J.P.: Intercellular adhesion and cell separation in plants. — Plant Cell Environ. 26: 977–989, 2003.
Jones, L., Seymour, G.B., Knox, J.P.: Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1→4)-β-D-galactan. — Plant Physiol. 113: 1405–1412, 1997.
Kikuchi, A., Edashige, Y., Ishii, T., Fujii, T., Satoh, S.: Variations in the structure of neutral sugar chains in the pectic polysaccharides of morphologically different carrot calli and correlations with the size of cell clusters. — Planta 198: 634–639, 1996.
Knox, J.P.: Cell adhesion, cell separation and plant morphogenesis. — Plant J. 2: 137–141, 1992.
Knox, J.P., Linstead, P.J., King, J., Cooper, C., Roberts, K.: Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. — Planta 181: 512–521, 1990.
Kohorn, B.D., Kobayashi, M., Johansen, S., Friedman, H.P., Fisher, A., Byers, N.: Wall-associated kinase 1 (WAK1) is crosslinked in endomembranes, and transport to the cell surface requires correct cell-wall synthesis. — J. Cell Sci. 119: 2282–2290, 2006.
Lai, K.S., Yusoff, K., Maziah, M.: Extracellular matrix as the early structural marker for Centella asiatica embryogenic tissues. — Biol. Plant. 55: 549–553, 2011.
Leboeuf, E., Thoiron, S., Lahaye, M.: Physico-chemical characteristics of cell walls from Arabidopsis thaliana microcalli showing different adhesion strengths. — J. exp. Bot. 55: 2087–2097, 2004.
Liners, F., Thibault, J.-F., Van Cutsem, P.: Influence of the degree of polymerization of oligogalacturonates and of esterification pattern of pectin on their recognition by monoclonal antibodies. — Plant Physiol. 99: 1099–1104, 1992.
McCartney, L., Knox, J.P.: Regulation of pectic polysaccharide domains in relation to cell development and cell properties in the pea testa. — J. exp. Bot. 53: 707–713, 2002.
McCartney, L., Ormerod, A.P., Gidley, M.J., Knox, J.P.: Temporal and spatial regulation of pectic (1→4)-β-D-galactan in cell walls of developing pea cotyledons: implications for mechanical properties. — Plant J. 22: 105–113, 2000.
McCartney, L., Steele-King, C.G., Jordan, E., Knox, J.P.: Cell wall pectic (1→4)-β-D-galactan marks the acceleration of cell elongation in the Arabidopsis seedling root meristem. — Plant J. 33: 447–454, 2003.
Micheli, F.: Pectin methylesterases: cell wall enzymes with important roles in plant physiology. — Trends Plant Sci. 6: 414–419, 2001.
Mingozzi, M., Morini, S., Lucchesini, M., Mensuali-Sodi, A.: Effects of leaf soluble sugars content and net photosynthetic rate of quince donor shoots on subsequent morphogenesis in leaf explants. — Biol. Plant. 55: 237–242, 2011.
Mohnen, D.: Biosynthesis of pectins and galactomannans. — In: Pinto, B.M. (ed.): Comprehensive Natural Products Chemistry. Vol. 3. Carbohydrates and Their Derivatives Including Tannins, Cellulose, and Related Lignins. Pp. 497–527. Elsevier, Oxford 1999.
Murashige, T., Skoog, F.: A revised medium for rapid growth and bio assays with tobacco tissue cultures. — Physiol. Plant. 15: 473–497, 1962.
Pan, X., Yang, X., Lin G., Zou, R., Chen, H., Samaj, J., Xu, C.: Ultrastructural changes and the distribution of arabinogalactan proteins during somatic embryogenesis of banana (Musa spp. AAA cv. ‘Yueyoukang 1’). — Physiol. Plant. 142: 372–389, 2011.
Pauly, M., Scheller, H.V.: O-Acetylation of plant cell wall polysaccharides: identification and partial characterization of a rhamnogalacturonan O-acetyl-transferase from potato suspension-cultured cells. — Planta 210: 659–667, 2000.
Pena, M.J., Carpita, N.C.: Loss of highly branched arabinans and debranching of rhamnogalacturonan I accompany loss of firm texture and cell separation during prolonged storage of apple. — Plant Physiol. 135: 1305–1313, 2004.
Popielarska-Konieczna, M., Kozieradzka-Kiszkurno, M., Świerczyńska, J., Góralski, G., Ślesak, H., Bohdanowicz, J.: Are extracellular matrix surface network components involved in signalling and protective function? — Plant Signal. Behav. 3: 707–709, 2008.
Ramirez, C., Chiancone, B. Testillano, P.S., Garcia-Fojeda, B., Germana, M.-A., Risueno, M.-C.: First embryogenic stages of Citrus microspore-derived embryos. — Acta Biol. cracov. Ser. Bot. 45: 53–58, 2003.
Ridley, B.L., O’Neill, M.A., Mohnen, D.: Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. — Phytochemistry 57: 929–967, 2001.
Rose, J.K.C. (ed.): The Plant Cell Wall. — Blackwell, Oxford 2003.
Satiat-Jeunemaitre, B., Hawes, C.: Immunocytochemistry for light microscopy. — In: Hawes, C., Satiat-Jeunemaitre, B. (ed.): Plant Cell Biology. A Practical Approach. Pp. 207–233. Oxford University Press, Oxford 2001.
Siedlecka, A., Wiklund, S., Peronne, M.-A., Micheli, F., Leśniewska, J., Sethson, I., Edlund, U., Richard, L., Sundberg, B., Mellerowicz, E.J.: Pectin methyl esterase inhibits intrusive and symplastic cell growth in developing wood cells of Populus. — Plant Physiol. 146: 554–565, 2008.
Sobry, S., Havelange, A., Van Cutsem, P.: Immunocytochemistry of pectins in shoot apical meristems: consequences for intercellular adhesion. — Protoplasma 225: 15–22, 2005.
Ulvskov, P., Wium, H., Bruce, D., Jorgensen, B., Qvist, K.B., Skjot, M., Hepworth, D., Borkhardt, B., Sorensen, S.O.: Biophysical consequences of remodeling the neutral side chains of rhamnogalacturonan I in tubers of transgenic potatoes. — Planta 220: 609–620, 2005.
Verhertbruggen, Y., Marcus, S.E., Haeger, A., Ordaz-Ortiz, J.J., Knox, J.P.: An extended set of monoclonal antibodies to pectic homogalacturonan. — Carbohydr. Res. 344: 1858–1862, 2009.
Vitha, S., Baluska, F., Jasik, J., Volkmann, D., Barlow, P.W.: Steedman’s wax for F-actin visualization. — In: Staiger, C.J., Baluska, F., Volkmann, D., Barlow, P.W. (ed.): Actin: a Dynamic Framework for Multiple Plant Cell Function. Pp. 619–636. Kluwer Academic Publishers, Dordrecht 2000.
Willats, W.G.T., Knox, J.P., Mikkelsen, J.D.: Pectin: new insights into an old polymer are starting to gel. — Trends Food Sci. Technol. 17: 97–104, 2006.
Willats, W.G.T., McCartney, L., Mackie, W., Knox, J.P.: Pectin: cell biology and prospects for functional analysis. — Plant mol. Biol. 47: 9–27, 2001.
Willats, W.G.T., Steele-King, C.G., Marcus, S.E., Knox, J.P.: Side chains of pectic polysaccharides are regulated in relation to cell proliferation and cell differentiation. — Plant J. 20: 619–628, 1999.
Wiśniewska, E., Majewska-Sawka, A.: The differences in cell wall composition in leaves and regenerating protoplasts of Beta vulgaris and Nicotiana tabacum.— Biol. Plant. 52: 634–641, 2008.
Wolf, S., Mouille, G., Pelloux, J.: Homogalacturonan methyl esterification and plant development. — Mol. Plant 2: 851–860, 2009.
Xu, C., Zhao, L., Pan, X., Samaj, J.: Developmental localization and methylesterification of pectin epitopes during somatic embryogenesis of banana (Musa spp. AAA). — PLoS ONE 6: e22992, 2011.
Zhang, G.F., Staehelin, L.A.: Functional compartmentation of the Golgi apparatus of plant cells. — Plant Physiol. 99: 1070–1083, 1992.
Zimmerman, J.L.: Somatic embryogenesis: a model for early development in higher plants. — Plant Cell 5: 1411–1423, 1993.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements: This work was partly supported by grant N303 092 32/3176 from the Polish Ministry of Science and Higher Education. We thank Prof. Paul Knox (Centre for Plant Sciences, University of Leeds, UK) for the generous gift of antibodies.
Rights and permissions
About this article
Cite this article
Sala, K., Potocka, I. & Kurczynska, E. Spatio-temporal distribution and methyl-esterification of pectic epitopes provide evidence of developmental regulation of pectins during somatic embryogenesis in Arabidopsis thaliana . Biol Plant 57, 410–416 (2013). https://doi.org/10.1007/s10535-013-0304-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10535-013-0304-6