Abstract
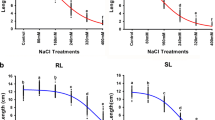
A quantitative trait loci (QTL) approach was applied to dissect the genetic control of the common wheat seedling response to osmotic stress. A set of 114 recombinant inbred lines was subjected to osmotic stress from the onset of germination to the 8th day of seedling development, induced by the presence of 12 % polyethylene glycol. Root, coleoptile and shoot length, and root/shoot length ratio were compared under stress and control conditions. In all, 35 QTL mapping to ten chromosomes, were identified. Sixteen QTL were detected in controls, 17 under stressed conditions, and two tolerance index QTL were determined. The majority of the QTL were not stress-specific. In regions on five chromosome arms (1AS, 1BL, 2DS, 5BL and 6BL) the QTL identified under stress co-mapped with QTL affecting the same trait in controls, and these were classified as seedling vigour QTL, in addition to those expressed in controls. Tolerance-related QTL were detected on four chromosome arms. A broad region on chromosome 1AL, including five QTL, with a major impact of the gene Glu-A1 (LOD 3.93) and marker locus Xksuh9d (LOD 2.91), positively affected root length under stress and tolerance index for root length, respectively. A major QTL (LOD 3.60), associated with marker locus Xcdo456a (distal part of chromosome arm 2BS) determined a tolerance index for shoot length. Three minor QTL (LOD < 3.0) for root length and root/shoot length ratio under osmotic stress were identified in the distal parts of chromosome arms 6DL (marker locus Xksud27a) and 7DL (marker locus Xksue3b). Selecting for the favourable alleles at marker loci associated with the detected QTL for growth traits may represent an efficient approach to enhance the plants’ ability to maintain the growth of roots, coleoptile and shoots in drought-prone soils at the critical early developmental stages.
Similar content being viewed by others
Abbreviations
- IA:
-
interval analysis
- Cl, Rl, Sl:
-
coleoptile, root and shoot length, respectively
- ITMI:
-
International Triticeae Mapping Initiative
- LOD:
-
logarithm of odds
- PEG:
-
polyethylene glycol
- QTL:
-
quantitative trait locus
- RFLP:
-
restriction fragment length polymorphism
- RILs:
-
recombinant inbred lines
- RSR:
-
root/shoot length ratio
- SMA:
-
single marker analysis
- SSR:
-
simple sequence repeats
- TI:
-
tolerance index
References
Almansouri, M., Kinet, J.-M., Lutts, S.: Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.).-Plant Soil 231: 243–254, 2001.
Anderson, J.A., Stack, R.W., Liu, S., Waldron, B.L., Fjeld, A.D., Coyne, C., Moreno-Sevilla, B., Mitchell Fetch, J., Song, Q.J., Cregan, P.B., Frohberg, R.C.: DNA markers for Fusarium head blight resistance QTLs in two wheat populations.-Theor. appl. Genet. 102: 1164–1168, 2001.
Bálint, A., Röder, M.S., Hell, R., Galiba, G., Börner, A.: Mapping of QTLs affecting copper tolerance and the Cu, Fe, Mn and Zn concentrations in the shoots of wheat seedlings.-Biol. Plant. 51: 129–134, 2007.
Bartels, D., Souer, E.: Molecular responses of higher plants to dehydration.-In: Hirt, H., Shinozaki, K. (ed.): Topics in Current Genetics. Vol. 4: Plant Responses to Abiotic Stress. Pp. 9–37. Springer-Verlag, Berlin-Heidelberg 2003.
Bewley, J.D.: Seed germination and dormancy.-Plant Cell 9: 1055–1066, 1997.
Blum, A.: Crop responses to drought and the interpretation of adaptation.-Plant Growth Regul. 20: 135–148, 1996.
Blum, A., Sinmena, B., Ziv, O.: An evaluation of seed and seedling drought tolerance screening tests in wheat.-Euphytica 29: 727–736, 1980.
Börner, A., Schumann, E., Fürste, A., Cöster, H., Leithold, B., Röder, M.S., Weber, W.E.: Mapping of quantitative trait loci determining agronomic important characters in hexaploid wheat (Triticum aestivum L.).-Theor. appl. Genet. 105: 921–936, 2002.
Botwright, T., Rebetzke, G., Condon, T., Richards, R.: The effect of rht genotype and temperature on coleoptile growth and dry matter partitioning in young wheat seedlings.-Aust. J. Plant Physiol.. 28: 417–423, 2001.
Cattivelli, L., Baldi, P., Crosatti, C., Di Fonzo, N., Faccioli, P., Grossi, M., Mastrangelo, A.M., Pecchioni, N., Stanca, A.M.: Chromosome regions and stress-related sequences involved in resistance to abiotic stress in Triticeae.-Plant mol. Biol. 48: 649–665, 2002.
Champoux, M.C., Wang, G., Sarkarung, S., Mackill, D.J., O’Toole, J.C., Huang, N., McCouch, S.R.: Locating genes associated with root morphology and drought avoidance in rice via linkage to molecular markers.-Theor. appl. Genet. 90: 969–981, 1995.
Collard, B.C.Y., Jahufer, M.Z.Z., Brouwer, J.B., Pang, E.C.K.: An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: the basic concepts.-Euphytica 142: 169–196, 2005.
Dhanda, S.S., Sethi, G.S., Behl, R.K.: Indices of drought tolerance in wheat genotypes at early stages of plant growth.-J. Agron. Crop Sci. 190: 6–12, 2004.
Faris, J.D., Friesen, T.L.: Identification of quantitative trait loci for race-nonspecific resistance to tan spot in wheat.-Theor. appl. Genet. 111: 386–392, 2005.
Galiba, G., Pecchioni, N., Vágújfalvi, A., Francia, E., Tóth, B., Barabaschi, D., Barilli, S., Crosatti, C., Cattivelli, L., Stanca, M.A.: Localization of QTLs and candidate genes involved in the regulation of frost resistance in cereals.-In: Tuberosa, R., Phillips, R.L., Gale, M.D. (ed.): Proc. Int. Congr. “In the Wake of the Double Helix: From the Green Revolution to the Gene Revolution”. Pp. 253–266. Avenue Media, Bologna 2005.
Kerepesi, I., Galiba, G.: Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings.-Crop Sci. 40: 482–487, 2000.
Liu, W.-J., Yuan, S., Zhang, N.-H., Lei, T., Duan, H.-G., Liang, H.-G., Lin, H.-H.: Effect of water stress on photosystem 2 in two wheat cultivars.-Biol. Plant. 50: 597–602, 2006.
Lohwasser, U., Röder, M.S., Börner, A.: QTL mapping of the domestication traits pre-harvest sprouting and dormancy in wheat (Triticum aestivum L.).-Euphytica 143: 247–249, 2005.
Marino, C.L., Nelson, J.C., Lu, Y.H., Sorrels, M.E., Leroy, P., Lopes, C.R., Hart, G.E.: RFLP-based linkage maps of the homeologous group 6 chromosomes of hexaploid wheat (Triticum aestivum L. Em. Thell.).-Genome 39: 359–366, 1996.
Matsui, T., Inanaga, S., Sugimoto, Y., Nakata, N.: Chromosomal location of genes controlling final coleoptile length in wheat using chromosome substitution lines.-Wheat Inform. Serv. 87: 22–26, 1998.
McCarty, D.R.: Genetic control and integration of maturation and germination pathways in seed development.-Annu. Rev. Plant Physiol. Plant mol. Biol. 46: 71–93, 1995.
Mujtaba, S.M., Khanzada, B., Ali, M., Naqvi, M.H., Mughal, S., Alam, S.M., Shirazi, M.U., Khan, M.A., Mumtaz, S.: The effect of polyethylene glycol on seed germination of wheat (Triticum aestivum L.) genotypes/lines.-Wheat Inform. Serv. 99: 58–60, 2005.
Nayyar, H.: Variation in osmoregulation in differentially drought-sensitive wheat genotypes involves calcium.-Biol. Plant. 47: 541–547, 2003/4.
Nelson, J.C.: QGene: software for marker-based genomic analysis and breeding.-Mol. Breed. 3: 239–245, 1997.
Nelson, J.C., Singh, R.P., Autrique, J.E., Sorrells, M.E.: Mapping genes conferring and suppressing leaf rust resistance in wheat.-Crop Sci. 37: 1928–1935, 1997.
Nelson, J.C., Sorrells, M.E., Van Deynze, A.E., Lu, Y.H., Atkinson, M., Bernard, M., Leroy, P., Faris, J.D., Anderson, A.: Molecular mapping of wheat: major genes and rearrangements in homoeologous groups 4, 5 and 7.-Genetics 141: 721–731, 1995a.
Nelson, J.C., Van Deynze, A.E., Autrique, E., Sorrells, M.E., Lu, Y.H., Merlino, M., Atkinson, M., Leroy, P.: Molecular mapping of wheat homoeologous group 2.-Genome 38: 516–524, 1995b.
Nelson, J.C., Van Deynze, A.E., Autrique, E., Sorrells, M.E., Lu, Y.H., Negre, S., Bernard, M., Leroy, P.: Molecular mapping of wheat homeologous group 3.-Genome 38: 525–533, 1995c.
Okçu, G., Kaya, M.D., Atak, M.: Effects of salt and drought stresses on germination and seedling growth of pea (Pisum sativum L.).-Turk. J. Agr. TÜBITAK 29: 237–242, 2005.
Perretant, M.R., Cadalen, T., Charmet, G., Sourdille, P., Nicolas, P., Boeuf, C., Tixier, M.H., Branlard, G., Bernard, S.: QTL analysis of bread-making quality in wheat using a doubled haploid population.-Theor. appl. Genet. 100: 1167–1175, 2000.
Pirdashti, H., Tahmasebi Sarvestani, Z., Nematzadeh, G.H., Ismail, A.: Effect of water stress on seed germination and seedling growth of rice (Oryza sativa L.) genotypes.-Pakistan J. Agron. 2: 217–222, 2003.
Rebetzke, G.J., Appels, R., Morrison, A.D., Richards, R.A., McDonald, G., Ellis, M.H., Spielmeyer, W., Bonnett, D.G.: Quantitative trait loci on chromosome 4B for coleoptile length and early vigour in wheat (Triticum aestivum L.).-Aust. J. agr. Res. 52: 1221–1234, 2001.
Reynolds, M.P., Skovmand, B., Trethowan, R.M., Pfeiffer, W.H.: Evaluating a conceptual model for drought tolerance.-In: Ribaut, J.M., Poland, D. (ed.): Molecular Approaches for the Genetic Improvement of Cereals for Stable Production in Water-Limited Environments. Pp. 49–53. CIMMYT, Mexico 2000.
Richards, R.A.: Defining selection criteria to improve yield under drought.-Plant Growth Regul. 20: 157–166, 1996.
Röder, M.S., Korzun, V., Wendehake, K., Plaschke, J., Tixier, M.-H., Leroy, P., Ganal, M.W.: A microsatellite map of wheat.-Genetics 149: 2007–2023, 1998.
Schmolke, M., Zimmermann, G., Buerstmayr, H., Schweizer, G., Miedaner, T., Korzun, V., Ebmeyer, E., Hartl, L.: Molecular mapping of Fusarium head blight resistance in the winter wheat population Dream/Lynx.-Theor. appl. Genet. 111: 747–756, 2005.
Shinozaki, K., Yamaguchi-Shinozaki, K., Liu, Q., Kasuda, M., Ichimura K., Mizogichi, T., Urao, T., Miyata, S., Nakashima, K., Shinwari, Z., Hiroshi, A., Sakuma, Y., Ito, T., Seki, M.: Molecular responses to drought stress in plants: regulation of gene expression and signal transduction.-In: Smallwood, M.F., Calvert, C.M., Bowles, D.J. (ed.): Plant Responses to Environmental Stress. Pp. 133–143. BIOS Scientific Publishers, Oxford 1999.
Simón, M.R., Ayala, F.M., Cordo, C.A., Röder, M.S., Börner, A.: Molecular mapping of quantitative trait loci determining resistance to Septoria tritici blotch caused by Mycosphaerella graminicola in wheat.-Euphytica 138: 41–48, 2004.
Sourdille, P., Perretant, M.R., Charmet, G., Leroy, P., Gautier, M.F., Joudrier, P., Nelson, J.C., Sorrells, M.E., Bernard, M.: Linkage between RFLP markers and genes affecting kernel hardness in wheat.-Theor. appl. Genet. 93: 580–586, 1996.
Sourdille, P., Snape, J.W., Cadalen, T., Charmet, G., Nakata, N., Bernard, S., Bernard, M.: Detection of QTLs for heading time and photoperiod response in wheat using a doubled-haploid population.-Genome 43: 487–494, 2000.
Tuberosa, R., Sanguineti, M.C., Landi, P., Giuliani, M.M., Salvi, S., Conti, S.: Identification of QTLs for root characteristics in maize grown in hydroponics and analysis of their overlap with QTLs for grain yield in the field at two water regimes.-Plant mol. Biol. 48: 697–712, 2002.
Van Deynze, A.E., Dubcovsky, J., Gill, K.S., Nelson, J.C., Sorrells, M.E., Dvorák, J., Gill, B.S., Lagudah, E.S., McCouch, S.R., Appels, R.: Molecular-genetic maps for group 1 chromosomes of Triticeae species and their relation to chromosomes in rice and oat.-Genome 38: 45–59, 1995.
Willenborg, C.J., Wildeman, J.C., Miller, A.K., Rossnagel, B.G., Shirtliffe, S.J.: Oat germination characteristics differ among genotypes, seed sizes, and osmotic potentials.-Crop Sci. 45: 2023–2029, 2005.
Worland, A.J., Börner, A., Korzun, V., Li, W.M., Petrovic, S., Sayers, E.J.: The influence of photoperiod genes on the adaptability of European winter wheats.-In: Braun, H.-J. (ed.): Wheat: Prospects for Global Improvement. Pp. 517–526. Kluwer Academic Publishers, Dordrecht 1997.
Worland, A., Korzun, V., Röder, M., Ganal, M.: Genetic analysis of the dwarfing gene Rht8 in wheat. Part II. The distribution and adaptive significance of allelic variants at the Rht8 locus of wheat as revealed by microsatellite screening.-Theor. appl. Genet. 96: 1110–1120, 1998.
Yordanov, I., Velikova, V., Tsonev, T.: Plant responses to drought and stress tolerance.-Bulg. J. Plant Physiol. 29 (Special Issue): 187–206, 2003.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Landjeva, S., Neumann, K., Lohwasser, U. et al. Molecular mapping of genomic regions associated with wheat seedling growth under osmotic stress. Biol Plant 52, 259–266 (2008). https://doi.org/10.1007/s10535-008-0056-x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10535-008-0056-x