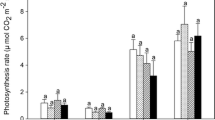
Abstract
Water relations were studied in Norway spruce [Picea abies (L.) Karst.] trees grown at ambient (AC, 350 μmol mol−1) and elevated (EC, 700 μmol mol−1) CO2 concentrations under temperate water stress. The results suggested that both crown position and variability in atmospheric CO2 concentration are responsible for different patterns of crown water relations. Mean hourly sap flux density (FSA) showed higher values in upper crown position in comparison with the whole crown in both AC and EC treatments. Mean soil-to-leaf hydraulic conductance (GTsa) was 1.4 times higher for the upper crown than that calculated across the whole crown for the trees in AC. However, GTsa did not vary significantly with crown position in EC trees, suggesting that elevated CO2 may mitigate differences in hydraulic supply for different canopy layers. The trees in EC treatment exhibited significantly higher values of FSA measured on the whole crown level and slightly higher soil water content compared to AC treatment, suggesting more economical use of soil water and therefore an advantage under water-limited conditions.
Similar content being viewed by others
Abbreviations
- CSoil :
-
soil water content
- FSA :
-
xylem sap flux expressed by sapwood transverse area, gs-stomatal conductance
- GT :
-
soil-to-leaf hydraulic conductance
- GTla :
-
soil-to-leaf hydraulic conductance expressed by projected leaf area
- GTsa :
-
soil-to-leaf hydraulic conductance expressed by sapwood transverse area
- PN :
-
net photosynthetic rate
- RWCW :
-
relative water content of sapwood
- VPD:
-
vapour pressure deficit
- ΨPd :
-
predawn shoot water potential
- ΨS :
-
soil water potential
- ΨX :
-
daily shoot water potential
References
Atwell, B.J., Henry, M.L., Whitehead, D.: Sapwood development in Pinus radiata trees grown for three years at ambient and elevated carbon dioxide partial pressures.-Tree Physiol. 23: 13–21, 2003.
Bond, B.J., Farnsworth, B.T., Coulombe, R.A., Winner, W.E.: Foliage physiology and biochemistry in response to light gradients in conifers with varying shade tolerance.-Oecologia 120: 183–192, 1999.
Brodribb, T.J., Holbrook, N.M., Gutierrez, M.V.: Hydraulic and photosynthetic co-ordination in seasonally dry tropical forest trees.-Plant Cell Environ. 25: 1435–1444, 2002.
Bunce, J.A.: Carbon dioxide effects on stomatal responses to the environment and water use by crops under field conditions.-Oecologia 140: 1–10, 2004.
Centritto, M., Magnani, F., Lee, H.S.J., Jarvis, P.G.: Interactive effects of elevated [CO2] and drought on cherry (Prunus avium) seedlings. II. Photosynthetic capacity and water relations.-New Phytol. 141: 141–154, 1999.
Ceulemans, R., Jach, M.E., Van De Velde, R., Lin, J.X., Stevens, M.: Elevated atmospheric CO2 alters wood production, wood quality and wood strength of Scots pine (Pinus sylvestris L.) after three years of enrichment.-Global Change Biol. 8: 153–162, 2002.
De Luis, I., Irigoyen, J.J., Sanchez-Diaz, M.: Elevated CO2 enhances plant growth in droughted N2-fixing alfalfa without improving water status.-Physiol. Plant. 107: 84–89, 1999.
Engel, V.C., Griffin, K.L., Murthy, R., Patterson, L., Klimas, C., Potosnak, M.: Growth CO2 concentration modifies the transpiration response of Populus deltoides to drought and vapor pressure deficit.-Tree Physiol. 24: 1137–1145, 2004.
Heath, J., Kerstiens, G.: Effects of elevated CO2 on leaf gas exchange in beech and oak at two levels of nutrient supply: consequences for sensitivity to drought in beech.-Plant Cell Environ. 20: 57–67, 1997.
Herrick, J.D., Maherali, H., Thomas, R.B.: Reduced stomatal conductance in sweetgum (Liquidambar styraciflua) sustained over long-term CO2 enrichment.-New Phytol. 162: 387–396, 2004.
Hubbard, R.M., Ryan, M.G., Stiller, V., Sperry, J.S.: Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine.-Plant Cell Environ. 24: 113–121, 2001.
Jarvis, A.J., Mansfield, T.A., Davies, W.J.: Stomatal behaviour, photosynthesis and transpiration under rising CO2.-Plant Cell Environ. 22: 639–648, 1999.
Jerez, M., Dean, T.J., Roberts, S.D., Evans, D.L.: Patterns of branch permeability with crown depth among loblolly pine families differing in growth rate and crown size.-Trees 18: 145–150, 2004.
Johnson, J.D., Tognetti, R., Paris, P.: Water relations and gas exchange in poplar and willow under water stress and elevated atmospheric CO2.-Physiol. Plant. 115: 93–100, 2002.
Köstner, B., Granier, A., Čermák, J.: Sapflow measurements in forest stands: methods and uncertainties.-Ann. Forest Sci. 55: 13–27, 1998.
Long, S.P.: Understanding the impacts of rising CO2: the contribution of environmental physiology.-In: Press, M.C., Scholes, J.D., Barcer, M.G. (ed.): Physiological Plant Ecology. Pp. 263–282. Blackwell Science, Oxford 1999.
Marek, M.V., Šprtová, M., Urban, O., Špunda, V.: Chlorophyll a fluorescence response of Norway spruce needles to the long-term effect of elevated CO2 in relation to their position within the canopy.-Photosynthetica 39: 437–455, 2001.
Marek, M.V., Urban, O., Šprtová, M., Pokorný, R., Rosová, Z., Kulhavý, J.: Photosynthetic assimilation of sun versus shade Norway spruce [Picea abies (L.) Karst] needles under the long-term impact of elevated CO2 concentration.-Photosynthetica 40: 259–267, 2002.
Mayr, S., Rothart, B., Dämon, B.: Hydraulic efficiency and safety of leader shoots and twigs in Norway spruce growing at the alpine timberline.-J. exp. Bot. 54: 2563–2568, 2003.
Medlyn, B.E., Barton, C.V.M., Broadmeadow, M,S.J., Ceulemans, R., De Angelis, P., Forstreuter, M., Freeman, M., Jackson, S.B., Kellomäki, S., Laitat, E., Rey, A., Roberntz, P., Sigurdsson, B.D., Strassemeyer, J., Wang, K., Curtis, P.S., Jarvis, P.G.: Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis.-New Phytol. 149: 247–264, 2001.
Meinzer, F.C.: Functional convergence in plant responses to the environment.-Oecologia 134: 1–11, 2003.
Niinemets, Ü., Kull, O., Tenhunen, J.D.: An analysis of light effects on foliar morphology, physiology, and light interception in temperate deciduous woody species of contrasting shade tolerance.-Tree Physiol. 18: 681–696, 1998.
Prichard, S.G., Rogers, H.H., Prior, S.A., Peterson, C.M.: Elevated CO2 and plant structure: a review.-Global Change Biol. 5: 807–837, 1999.
Protz, C.G., Silins, U., Lieffers, V.J.: Reduction in branch sapwood hydraulic permeability as a factor limiting survival of lower branches of lodgepole pine.-Can. J. Forest Res. 30: 1088–1095, 2000.
Santiago, L.S., Goldstein, G., Meinzer, F.C., Fisher, J.B., Machado, K., Woodruff, D., Jones, T.: Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees.-Oecologia 140: 543–550, 2004.
Saxe, H., Ellsworth, D.S., Heath, J.: Tree and forest functioning in an enriched CO2 atmosphere.-New Phytol. 139: 395–436, 1998.
Schulte, M., Herschbach, C., Rennenberg, H.: Interactive effects of elevated atmospheric CO2, mycorrhization and drought on long-distance transport of reduced sulphur in young pedunculate oak trees (Quercus robur L.).-Plant Cell Environ. 21: 917–926, 1998.
Schäfer, K.V.R., Oren, R., Lai, C., Katul, G.G.: Hydrologic balance in an intact temperate forest ecosystem under ambient and elevated atmospheric CO2 concentration.-Global Change Biol. 8: 895–911, 2002.
Sellin, A., Kupper, P.: Within-crown variation in leaf conductance of Norway spruce: effects of irradiance, vapour pressure deficit, leaf water status and plant hydraulic constraints.-Ann. Forest Sci. 61: 419–429, 2004.
Sellin, A., Kupper, P.: Effects of light availability versus hydraulic constraints on stomatal responses within a crown of silver birch.-Oecologia 142: 388–397, 2005.
Tognetti, R., Longobucco, A., Miglietta, F., Rashi, A.: Transpiration and stomatal behaviour of Quercus ilex plants during the summer in a Mediterranean carbon dioxide spring.-Plant Cell Environ. 21: 613–622, 1998.
Tognetti, R., Longobucco, A., Miglietta, F., Rashi, A.: Water relations, stomatal response and transpiration of Quercus pubescens trees during summer in a Mediterranean carbon dioxide spring.-Tree Physiol. 19: 261–270, 1999.
Tognetti, R., Peñuelas, J.: Nitrogen and carbon concentrations, and stable isotope ratios in Mediterranean shrubs growing in the proximity of a CO2 spring.-Biol. Plant. 46: 411–418, 2003.
Tognetti, R., Raschi, A., Jones, M.B.: Seasonal patterns of tissue water relations in three Mediterranean shrubs cooccurring at a natural CO2 spring.-Plant Cell Environ. 23: 1341–1351, 2000.
Tyree, M.T., Ewers, F.W.: The hydraulic architecture of trees and other woody plants.-New Phytol. 119: 345–360, 1991.
Urban, O., Janouš, D., Pokorný, R., Marková, I., Pavelka, M., Fojtík, Z., Šprtová, M., Kalina, J., Marek, M.V.: Glass domes with adjustable windows: A novel technique for exposing juvenile forest stands to elevated CO2 concentration.-Photosynthetica 39: 395–401, 2001.
Whitehead, D.: Regulation of stomatal conductance and transpiration in forest canopies.-Tree Physiol. 18: 633–644, 1998.
Wullschleger, S.D., Norby, R.J.: Sap velocity and canopy transpiration in a sweetgum stand exposed to free-air CO2 enrichment (FACE).-New Phytol. 150: 489–498, 2001.
Wullschleger, S.D., Tchaplinski, T.J., Norby, R.J.: Plant water relations at elevated CO2-implications for water-limited environments.-Plant Cell Environ. 25: 319–331, 2002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kupper, P., Sellin, A., Klimánková, Z. et al. Water relations in Norway spruce trees growing at ambient and elevated CO2 concentrations. Biol Plant 50, 603–609 (2006). https://doi.org/10.1007/s10535-006-0095-0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10535-006-0095-0