Abstract
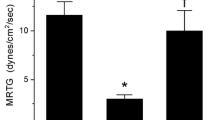
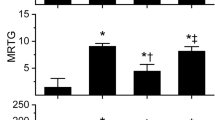
Envenomation by vipers with hemotoxic enzymes continues to be a worldwide source of morbidity and mortality. The present work examined the effects of exposure of venom enzymes to carbon monoxide and O-phenylhydroxylamine, agents that modulate the biometal heme, by forming carboxyheme and metheme, respectively. Four venoms obtained from medically important, diverse snake venom found in Africa, Asia and Australia were analyzed. The species that had venom tested in human plasma with thrombelastography and heme modulating agents were Deinagkistrodon acutus, Protobothrops mucrosquamatus, Dispholidus typus and Pseudonaja textilis. These venoms varied four hundred-fold in potency (ng-µg/ml) to exert procoagulant effects on human plasma; further, there was species specific variability in venom inhibition after exposure to carboxyheme or metheme agents. Lastly, using a wide range of carbon monoxide concentrations, it was determined that the factor V component of P. textilis venom was likely inhibited before the factor X component. Further investigation using this thrombelastograph-based, venom “kinetomic” methodology involving heme modulation will demonstrate in time its laboratory and clinical utility.




Similar content being viewed by others
References
Debono J, Dobson J, Casewell NR, Romilio A, Li B, Kurniawan N, Mardon K, Weisbecker V, Nouwens A, Kwok HF, Fry BG (2017) Coagulating colubrids: evolutionary, pathophysiological and biodiscovery implications of venom variations between Boomslang (Dispholidus typus) and Twig Snake (Thelotornis mossambicanus). Toxins (Basel). https://doi.org/10.3390/toxins9050171
Hiestand PC, Hiestand RR (1979) Dispholidus typus (boomslang) snake venom: purification and properties of the coagulant principle. Toxicon 17:489–498
Li A, Zhang C, Wang J, Wang J, Jiang H, Li J, Ma X, Zhang W, Lu Y (2018) Cloning, expression, purification and bioactivity evaluation of a thrombin-like enzyme from Deinagkistrodon acutus venom gland library. Biotechnol Lett 40:93–102
Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ (2002) Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res 90:E17–E24
Nielsen VG (2018) Crotalus atrox venom exposed to carbon monoxide has decreased fibrinogenolytic activity in vivo in rabbits. Basic Clin Pharmacol Toxicol 122:82–86
Nielsen VG, Garza JI (2014) Comparison of the effects of CORM-2, CORM-3 and CORM-A1 on coagulation in human plasma. Blood Coagul Fibrinolysis 25:801–805
Nielsen VG, Cerruti MA, Valencia OM, Amos Q (2016) Decreased snake venom metalloproteinase effects via inhibition of enzyme and modification of fibrinogen. Biometals 29:913–919
Nielsen VG, Frank N, Matika RW (2018) Carbon monoxide inhibits hemotoxic activity of Elapidae venoms: potential role of heme. Biometals 31:51–59
Steen M (2002) Factor Va-factor Xa interactions: molecular sites involved in enzyme:cofactor assembly. Scand J Clin Lab Invest Suppl 237:5–11
Stocker K, Hauer H, Müller C, Triplett DA (1994) Isolation and characterization of Textarin, a prothrombin activator from eastern brown snake (Pseudonaja textilis) venom. Toxicon 32:1227–1236
Suntravat M, Langlais PR, Sánchez EE, Nielsen VG (2018) CatroxMP-II: a heme-modulated fibrinogenolytic metalloproteinase isolated from Crotalus atrox venom. Biometals, in press. https://doi.org/10.1007/s10534-018-0107-5
Viala VL, Hildebrand D, Trusch M, Fucase TM, Sciani JM, Pimenta DC, Arni RK, Schlüter H, Betzel C, Mirtschin P, Dunstan N, Spencer PJ (2015) Venomics of the Australian eastern brown snake (Pseudonaja textilis): Detection of new venom proteins and splicing variants. Toxicon 107(Pt B):252–265
Villalta M, Pla D, Yang SL, Sanz L, Segura A, Vargas M, Chen PY, Herrera M, Estrada R, Cheng YF, Lee CD, Cerdas M, Chiang JR, Angulo Y, León G, Calvete JJ, Gutiérrez JM (2012) Snake venomics and antivenomics of Protobothrops mucrosquamatus and Viridovipera stejnegeri from Taiwan: keys to understand the variable immune response in horses. J Proteomics 22(75):5628–5645
Wang S, Xu X, Gao S, Zhu S, Rong R, Li B (2014) Purification and partial characterization of a novel fibrinogenase from the venom of Deinagkistrodon acutus: inhibition of platelet aggregation. Protein Expr Purif 99:99–105
Acknowledgements
This investigation was supported by the Department of Anesthesiology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose except that Mr. Frank is the owner of Mtoxins.
Rights and permissions
About this article
Cite this article
Nielsen, V.G., Frank, N. Differential heme-mediated modulation of Deinagkistrodon, Dispholidus, Protobothrops and Pseudonaja hemotoxic venom activity in human plasma. Biometals 31, 951–959 (2018). https://doi.org/10.1007/s10534-018-0137-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-018-0137-z