Abstract
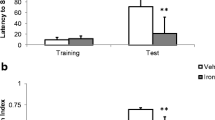
Brain-derived neurotrophic factor (BDNF) plays a key role in neural development and physiology, as well as in pathological states. Post-mortem studies demonstrate that BDNF is reduced in the brains of patients affected by neurodegenerative diseases. Iron accumulation has also been associated to the pathogenesis of neurodegenerative diseases. In rats, iron overload induces persistent memory deficits, increases oxidative stress and apoptotic markers, and decreases the expression of the synaptic marker, synaptophysin. Deferiprone (DFP) is an oral iron chelator used for the treatment of systemic iron overload disorders, and has recently been tested for Parkinson’s disease. Here, we investigated the effects of iron overload on BDNF levels and on mRNA expression of genes encoding TrkB, p75NTR, catalase (CAT) and NQO1. We also aimed at investigating the effects of DFP on iron-induced impairments. Rats received iron or vehicle at postnatal days 12–14 and when adults, received chronic DFP or water (vehicle). Recognition memory was tested 19 days after the beginning of chelation therapy. BDNF measurements and expression analyses in the hippocampus were performed 24 h after the last day of DFP treatment. DFP restored memory and increased hippocampal BDNF levels, ameliorating iron-induced effects. Iron overload in the neonatal period reduced, while treatment with DFP was able to rescue, the expression of antioxidant enzymes CAT and NQO1.




Similar content being viewed by others
References
Agrawal S, Fox J, Thyagarajan B, Fox JH (2018) Brain mitochondrial iron accumulates in Huntington’s disease, mediates mitochondrial dysfunction, and can be removed pharmacologically. Free Radic Biol Med 120:317–329. https://doi.org/10.1016/j.freeradbiomed.2018.04.002
Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK (2013) GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther 138:155–175. https://doi.org/10.1016/j.pharmthera.2013.01.004
Balaratnasingam S, Janca A (2012) Brain derived neurotrophic factor: a novel neurotrophin involved in psychiatric and neurological disorders. Pharmacol Ther 134:116–124. https://doi.org/10.1016/j.pharmthera.2012.01.006
Bar-Am O, Amit T, Kupershmidt L, Aluf Y, Mechlovich D, Kabha H, Danovitch L, Zurawski VR, Youdim MB, Weinreb O (2015) Neuroprotective and neurorestorative activities of a novel iron chelator-brain selective monoamine oxidase-A/monoamine oxidase-B inhibitor in animal models of Parkinson’s disease and aging. Neurobiol Aging 36:1529–1542. https://doi.org/10.1016/j.neurobiolaging.2014.10.026
Bauernfeind AL, Babbitt CC (2017) The predictive nature of transcript expression levels on protein expression in adult human brain. BMC Genomics 18:322. https://doi.org/10.1186/s12864-017-3674-x
Bekinschtein P, Cammarota M, Medina JH (2014) BDNF and memory processing. Neuropharmacology 76:677–683. https://doi.org/10.1016/j.neuropharm.2013.04.024
Belaidi AA, Bush AI (2016) Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J Neurochem 139(Suppl 1):179–197. https://doi.org/10.1111/jnc.13425
Bonefeld BE, Elfving B, Wegener G (2008) Reference genes for normalization: a study of rat brain tissue. Synapse 62:302–309. https://doi.org/10.1002/syn.20496
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bustin SA, Benes V, Garson J et al (2013) The need for transparency and good practices in the qPCR literature. Nat Methods 10:1063–1067. https://doi.org/10.1038/nmeth.2697
Carboni E, Tatenhorst L, Tönges L, Barski E, Dambeck V, Bähr M, Lingor P (2017) Deferiprone rescues behavioral deficits induced by mild iron exposure in a mouse model of alpha-synuclein aggregation. Neuromol Med 19(2–3):309–321. https://doi.org/10.1007/s12017-017-8447-9
Choi S, Friedman WJ (2014) Interleukin-1β enhances neuronal vulnerability to proNGF-mediated apoptosis by increasing surface expression of p75(NTR) and sortillin. Neuroscience 257:11–19. https://doi.org/10.1016/j.neuroscience.2013.10.058
da Silva VK, de Freitas BS, da Silva AD, Nery LR, Falavigna L, Ferreira RD, Bogo MR, Hallak JE, Zuardi AW, Crippa JA, Schröder N (2014) Cannabidiol normalizes caspase 3, synaptophysin, and mitochondrial fission protein DNM1L expression levels in rats with brain iron overload: implications for neuroprotection. Mol Neurobiol 49:222–233. https://doi.org/10.1007/s12035-013-8514-7
de Lima MN, Polydoro M, Laranja DC, Bonatto F, Bromberg E, Moreira JC, Dal-Pizzol F, Schröder N (2005) Recognition memory impairment and brain oxidative stress induced by postnatal iron administration. Eur J Neurosci 21:2521–2528. https://doi.org/10.1111/j.1460-9568.2005.04083.x
de Lima MN, Luft T, Roesler R, Schröder N (2006) Temporary inactivation reveals an essential role of the dorsal hippocampus in consolidation of object recognition memory. Neurosci Lett 405(1–2):142–146. https://doi.org/10.1016/j.neulet.2006.06.044
de Lima MN, Presti-Torres J, Caldana F, Grazziotin MM, Scalco FS, Guimarães MR, Bromberg E, Franke SI, Henriques JA, Schröder N (2007) Desferoxamine reverses neonatal iron-induced recognition memory impairment in rats. Eur J Pharmacol 570:111–114. https://doi.org/10.1016/j.ejphar.2007.06.002
de Lima MN, Dias CP, Torres JP, Dornelles A, Garcia VA, Scalco FS, Guimarães MR, Petry RC, Bromberg E, Constantino L, Budni P, Dal-Pizzol F, Schröder N (2008) Reversion of age-related recognition memory impairment by iron chelation in rats. Neurobiol Aging 29:1052–1059. https://doi.org/10.1016/j.neurobiolaging.2007.02.006
Devos D, Moreau C, Devedjian JC, Kluza J, Petrault M, Laloux C, Jonneaux A, Ryckewaert G, Garçon G, Rouaix N, Duhamel A, Jissendi P, Dujardin K, Auger F, Ravasi L, Hopes L, Grolez G, Firdaus W, Sablonnière B, Strubi-Vuillaume I, Zahr N, Destée A, Corvol JC, Pöltl D, Leist M, Rose C, Defebvre L, Marchetti P, Cabantchik ZI, Bordet R (2014) Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid Redox Signal 21:195–210. https://doi.org/10.1089/ars.2013.5593
Ding B, Chen KM, Ling HW, Sun F, Li X, Wan T, Chai WM, Zhang H, Zhan Y, Guan YJ (2009) Correlation of iron in the hippocampus with MMSE in patients with Alzheimer’s disease. J Magn Reson Imaging 29:793–798. https://doi.org/10.1002/jmri.21730
Dusek P, Schneider SA, Aaseth J (2016) Iron chelation in the treatment of neurodegenerative diseases. J Trace Elem Med Biol 38:81–92. https://doi.org/10.1016/j.jtemb.2016.03.010
Fagherazzi EV, Garcia VA, Maurmann N, Bervanger T, Halmenschlager LH, Busato SB, Hallak JE, Zuardi AW, Crippa JA, Schröder N (2012) Memory-rescuing effects of cannabidiol in an animal model of cognitive impairment relevant to neurodegenerative disorders. Psychopharmacology 219:1133–1140. https://doi.org/10.1007/s00213-011-2449-3
Fernandez LL, de Lima MN, Scalco F, Vedana G, Miwa C, Hilbig A, Vianna M, Schröder N (2011) Early post-natal iron administration induces astroglial response in the brain of adult and aged rats. Neurotox Res 20:193–199. https://doi.org/10.1007/s12640-010-9235-6
Figueiredo LS, de Freitas BS, Garcia VA, Dargél VA, Köbe LM, Kist LW, Bogo MR, Schröder N (2016) Iron loading selectively increases hippocampal levels of ubiquitinated proteins and impairs hippocampus-dependent memory. Mol Neurobiol 53:6228–6239. https://doi.org/10.1007/s12035-015-9514-6
Fine JM, Baillargeon AM, Renner DB, Hoerster NS, Tokarev J, Colton S, Pelleg A, Andrews A, Sparley KA, Krogh KM, Frey WH, Hanson LR (2012) Intranasal deferoxamine improves performance in radial arm water maze, stabilizes HIF-1α, and phosphorylates GSK3β in P301L tau transgenic mice. Exp Brain Res 219:381–390. https://doi.org/10.1007/s00221-012-3101-0
Gao XP, Liu Q, Nair B, Wong-Riley MT (2014) Reduced levels of brain-derived neurotrophic factor contribute to synaptic imbalance during the critical period of respiratory development in rats. Eur J Neurosci 40:2183–2195. https://doi.org/10.1111/ejn.12568
Guo C, Wang T, Zheng W, Shan ZY, Teng WP, Wang ZY (2013) Intranasal deferoxamine reverses iron-induced memory deficits and inhibits amyloidogenic APP processing in a transgenic mouse model of Alzheimer’s disease. Neurobiol Aging 34:562–575. https://doi.org/10.1016/j.neurobiolaging.2012.05.009
Guo C, Zhang YX, Wang T, Zhong ML, Yang ZH, Hao LJ, Chai R, Zhang S (2015) Intranasal deferoxamine attenuates synapse loss via up-regulating the P38/HIF-1α pathway on the brain of APP/PS1 transgenic mice. Front Aging Neurosci 7:104. https://doi.org/10.3389/fnagi.2015.00104
Heldt SA, Stanek L, Chhatwal JP, Ressler KJ (2007) Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatr 12:656–670. https://doi.org/10.1038/sj.mp.4001957
Huerta-García E, Pérez-Arizti JA, Márquez-Ramírez SG, Delgado-Buenrostro NL, Chirino YI, Iglesias GG, López-Marure R (2014) Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radic Biol Med 73:84–94. https://doi.org/10.1016/j.freeradbiomed.2014.04.026
Kakhlon O, Breuer W, Munnich A, Cabantchik ZI (2010) Iron redistribution as a therapeutic strategy for treating diseases of localized iron accumulation. Can J Physiol Pharmacol 88:187–196. https://doi.org/10.1139/Y09-128
Kemppainen S, Rantamäki T, Jerónimo-Santos A, Lavasseur G, Autio H, Karpova N, Kärkkäinen E, Stavén S, Vicente Miranda H, Outeiro TF, Diógenes MJ, Laroche S, Davis S, Sebastião AM, Castrén E, Tanila H (2012) Impaired TrkB receptor signaling contributes to memory impairment in APP/PS1 mice. Neurobiol Aging. https://doi.org/10.1016/j.neurobiolaging.2011.11.006
Kupershmidt L, Weinreb O, Amit T, Mandel S, Bar-Am O, Youdim MB (2011) Novel molecular targets of the neuroprotective/neurorescue multimodal iron chelating drug M30 in the mouse brain. Neuroscience 189:345–358. https://doi.org/10.1016/j.neuroscience.2011.03.040
Langkammer C, Ropele S, Pirpamer L, Fazekas F, Schmidt R (2014) MRI for iron mapping in Alzheimer’s disease. Neurodegener Dis 13:189–191. https://doi.org/10.1159/000353756
Langley J, Huddleston DE, Sedlacik J, Boelmans K, Hu XP (2017) Parkinson’s disease-related increase of T2*-weighted hypointensity in substantia nigra pars compacta. Mov Disord 32(3):441–449. https://doi.org/10.1002/mds.26883
Lei P, Ayton S, Appukuttan AT, Volitakis I, Adlard PA, Finkelstein DI, Bush AI (2015) Clioquinol rescues Parkinsonism and dementia phenotypes of the tau knockout mouse. Neurobiol Dis 81:168–175. https://doi.org/10.1016/j.nbd.2015.03.015
Li K, Reichmann H (2016) Role of iron in neurodegenerative diseases. J Neural Transm 123:389–399. https://doi.org/10.1007/s00702-016-1508-7
Li Y, Pan K, Chen L, Ning JL, Li X, Yang T, Terrando N, Gu J, Tao G (2016) Deferoxamine regulates neuroinflammation and iron homeostasis in a mouse model of postoperative cognitive dysfunction. J Neuroinflammation 13:268. https://doi.org/10.1186/s12974-016-0740-2
Longo FM, Yang T, Knowles JK, Xie Y, Moore LA, Massa SM (2007) Small molecule neurotrophin receptor ligands: novel strategies for targeting Alzheimer’s disease mechanisms. Curr Alzheimer Res 4:503–506. https://doi.org/10.2174/156720507783018316
Ma Y, Zhou T, Kong X, Hider RC (2012) Chelating agents for the treatment of systemic iron overload. Curr Med Chem 19:2816–2827. https://doi.org/10.2174/092986712800609724
Mattson MP (2008) Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann NY Acad Sci 1144:97–112. https://doi.org/10.1196/annals.1418.005
Minichiello L (2009) TrkB signalling pathways in LTP and learning. Nat Rev Neurosci 10:850–860. https://doi.org/10.1038/nrn2738
Miwa CP, de Lima MN, Scalco F, Vedana G, Mattos R, Fernandez LL, Hilbig A, Schröder N, Vianna MR (2011) Neonatal iron treatment increases apoptotic markers in hippocampal and cortical areas of adult rats. Neurotox Res 19:527–535. https://doi.org/10.1007/s12640-010-9181-3
Nagahara AH, Tuszynski MH (2011) Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov 10:209–219. https://doi.org/10.1038/nrd3366
Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH (2009) Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med 15:331–337. https://doi.org/10.1038/nm.1912
Pan K, Li X, Chen Y, Zhu D, Li Y, Tao G, Zuo Z (2016) Deferoxamine pre-treatment protects against postoperative cognitive dysfunction of aged rats by depressing microglial activation via ameliorating iron accumulation in hippocampus. Neuropharmacology 111:180–194. https://doi.org/10.1016/j.neuropharm.2016.09.004
Penke L, Valdés Hernandéz MC, Maniega SM, Gow AJ, Murray C, Starr JM, Bastin ME, Deary IJ, Wardlaw JM (2012) Brain iron deposits are associated with general cognitive ability and cognitive aging. Neurobiol Aging 33:510–517. https://doi.org/10.1016/j.neurobiolaging.2010.04.032
Pinheiro RM, de Lima MN, Portal BC, Busato SB, Falavigna L, Ferreira RD, Paz AC, de Aguiar BW, Kapczinski F, Schröder N (2015) Long-lasting recognition memory impairment and alterations in brain levels of cytokines and BDNF induced by maternal deprivation: effects of valproic acid and topiramate. J Neural Transm 122:709–719. https://doi.org/10.1007/s00702-014-1303-2
Pláteník J, Fišar Z, Buchal R, Jirák R, Kitzlerová E, Zvěřová M, Raboch J (2014) GSK3β, CREB, and BDNF in peripheral blood of patients with Alzheimer’s disease and depression. Prog Neuropsychopharmacol Biol Psychiatry 50:83–93. https://doi.org/10.1016/j.pnpbp.2013.12.001
Rodrigue KM, Daugherty AM, Haacke EM, Raz N (2013) The role of hippocampal iron concentration and hippocampal volume in age-related differences in memory. Cereb Cortex 23:1533–1541. https://doi.org/10.1093/cercor/bhs139
Schröder N, Fredriksson A, Vianna MR, Roesler R, Izquierdo I, Archer T (2001) Memory deficits in adult rats following postnatal iron administration. Behav Brain Res 124:77–85. https://doi.org/10.1016/S0166-4328(01)00236-4
Schröder N, Figueiredo LS, de Lima MN (2013) Role of brain iron accumulation in cognitive dysfunction: evidence from animal models and human studies. J Alzheimers Dis 34:797–812. https://doi.org/10.3233/JAD-121996
Silva PF, Garcia VA, da Dornelles AS, Silva VK, Maurmann N, Portal BC, Ferreira RD, Piazza FC, Roesler R, Schröder N (2012) Memory impairment induced by brain iron overload is accompanied by reduced H3K9 acetylation and ameliorated by sodium butyrate. Neuroscience 200:42–49. https://doi.org/10.1016/j.neuroscience.2011.10.038
Sofic E, Salkovic-Petrisic M, Tahirovic I, Sapcanin A, Mandel S, Youdim M, Riederer P (2015) Brain catalase in the streptozotocin-rat model of sporadic Alzheimer’s disease treated with the iron chelator-monoamine oxidase inhibitor, M30. J Neural Transm 122(4):559–564. https://doi.org/10.1007/s00702-014-1307-y
Sripetchwandee J, Pipatpiboon N, Chattipakorn N, Chattipakorn S (2014) Combined therapy of iron chelator and antioxidant completely restores brain dysfunction induced by iron toxicity. PLoS ONE 9:e85115. https://doi.org/10.1371/journal.pone.0085115
Tanila H (2017) The role of BDNF in Alzheimer’s disease. Neurobiol Dis 97:114–118. https://doi.org/10.1016/j.nbd.2016.05.008
Wang G, Hu W, Tang Q, Wang L, Sun XG, Chen Y, Yin Y, Xue F, Sun Z (2016) Effect comparison of both iron chelators on outcomes, iron deposit, and iron transporters after intracerebral hemorrhage in rats. Mol Neurobiol 53:3576–3585. https://doi.org/10.1007/s12035-015-9302-3
Warburton EC, Brown MW (2015) Neural circuitry for rat recognition memory. Behav Brain Res 285:131–139. https://doi.org/10.1016/j.bbr.2014.09.050
Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13:1045–1060. https://doi.org/10.1016/S1474-4422(14)70117-6
Wu Q, Zhang XS, Wang HD, Zhang X, Yu Q, Li W, Zhou ML, Wang XL (2014) Astaxanthin activates Nuclear Factor Erythroid-Related Factor 2 and the Antioxidant Responsive Element (Nrf2-ARE) pathway in the brain after subarachnoid hemorrhage in rats and attenuates early brain injury. Mar Drugs 12:6125–6141. https://doi.org/10.3390/md12126125
Zhang L, Hu R, Li M, Li F, Meng H, Zhu G, Lin J, Feng H (2013) Deferoxamine attenuates iron-induced long-term neurotoxicity in rats with traumatic brain injury. Neurol Sci 34:639–645. https://doi.org/10.1007/s10072-012-1090-1
Zhang HY, Song N, Jiang H, Bi MX, Xie JX (2014) Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor inhibit ferrous iron influx via divalent metal transporter 1 and iron regulatory protein 1 regulation in ventral mesencephalic neurons. Biochim Biophys Acta 1843:2967–2975. https://doi.org/10.1016/j.bbamcr.2014.09.010
Zhang XY, Cao JB, Zhang LM, Li YF, Mi WD (2015) Deferoxamine attenuates lipopolysaccharide-induced neuroinflammation and memory impairment in mice. J Neuroinflam 12:20. https://doi.org/10.1186/s12974-015-0238-3
Zhao L, Hadziahmetovic M, Wang C, Xu X, Song Y, Jinnah HA, Wodzinska J, Iacovelli J, Wolkow N, Krajacic P, Weissberger AC, Connelly J, Spino M, Lee MK, Connor J, Giasson B, Harris ZL, Dunaief JL (2015) Cp/Heph mutant mice have iron-induced neurodegeneration diminished by deferiprone. J Neurochem 135:958–974. https://doi.org/10.1111/jnc.13292
Zucca FA, Segura-Aguilar J, Ferrari E, Muñoz P, Paris I, Sulzer D, Sarna T, Casella L, Zecca L (2017) Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol 155:96–119. https://doi.org/10.1016/j.pneurobio.2015.09.012
Funding
This research was supported by the National Council for Scientific and Technological Development (CNPq; Grant Numbers 308290/2015-1and 421643/2016-1 to NS); the National Institute for Translational Medicine (INCT-TM). M.R.B. is Research Career Awarded of the CNPq. V.C.D. is recipient of a PROBIC/FAPERGS fellowship. P. C. de F. C. and R.T.M. are recipients of BPA/PUCRS scholarships. L.W.K. is recipient of fellowship CAPES/PNPD Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted and approved by the Institutional Ethics Committee (SIPESQ# 7205).
Rights and permissions
About this article
Cite this article
Alcalde, L.A., de Freitas, B.S., Machado, G.D.B. et al. Iron chelator deferiprone rescues memory deficits, hippocampal BDNF levels and antioxidant defenses in an experimental model of memory impairment. Biometals 31, 927–940 (2018). https://doi.org/10.1007/s10534-018-0135-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-018-0135-1