Abstract
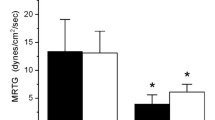
Iron-bound fibrinogen has been noted to accelerate plasmatic coagulation in patients with divergent conditions involving upregulation of heme oxygenase activity, including hemodialysis, Alzheimer’s disease, sickle cell anemia, and chronic migraine. Our goal was to determine if a site of iron-fibrinogen interaction was on the alpha chain. Using thrombelastography, we compared the coagulation kinetic profiles of plasma exposed to 0–10 µM ferric chloride after activation of coagulation with thrombin generated by contact activation of plasma with the plastic sample cup or by exposure to 1 µg/ml of Calloselasma rhodostoma venom (rich in ancrod activity), which causes coagulation via polymerization of alpha chain monomers. Venom mediated coagulation always occurred before thrombin activated thrombus formation, and ferric chloride always diminished the time of onset of coagulation and increased the velocity of clot growth. Iron enhances plasmatic coagulation kinetics by modulating the alpha chain of fibrinogen.


Similar content being viewed by others
References
Bell WR, Shapiro SS, Martinez J, Nossel HL (1978) The effects of ancrod, the coagulating enzyme from the venom of Malayan pit viper (A. rhodostoma) on prothrombin and fibrinogen metabolism and fibrinopeptide A release in man. J Lab Clin Med 91:592–604
Burney PR, White N, Pfaendtner J (2014) Structural effects of methionine oxidation on isolated subdomains of human fibrin D and αC regions. PLoS One 27(9):e86981
Dempfle CE, Argiriou S, Kucher K, Müller-Peltzer H, Rübsamen K, Heene DL (2000) Analysis of fibrin formation and proteolysis during intravenous administration of ancrod. Blood 96:2793–2802
Dempfle CE, Argiriou S, Alesci S, Kucher K, Müller-Peltzer H, Rübsamen K, Heene DL (2001) Fibrin formation and proteolysis during ancrod treatment. Evidence for des-A-profibrin formation and thrombin independent factor XIII activity. Ann N Y Acad Sci 936:210–214
Lipinski B, Pretorius E (2013) Iron-induced fibrin in cardiovascular disease. Curr Neurovasc Res 10:269–274
Matika RW, Nielsen VG, Steinbrenner EB, Sussman AN, Madhrira M (2014) Hemodialysis patients have plasmatic hypercoagulability and decreased fibrinolytic vulnerability: role of carbon monoxide. ASAIO J 60:716–721
Nielsen VG, Pretorius E (2014a) Iron and carbon monoxide enhance coagulation and attenuate fibrinolysis by different mechanisms. Blood Coagul Fibrinolysis 25:695–702
Nielsen VG, Pretorius E (2014b) Iron enhanced coagulation is attenuated by chelation: a thrombelastographic and ultrastructural analysis. Blood Coagul Fibrinolysis 25:845–850
Nielsen VG, Kulin W, LaWall JS, MacFarland FN, Chen A, Hadley HA, DaDeppo AJ, Steinbrenner EB, Matika RW (2015a) Chronic migraineurs form circulating carboxyhemefibrinogen and iron-bound fibrinogen. CNS Neurol Disord Drug Targets 14:1079–1085
Nielsen VG, Pretorius E, Jacobsen WK, Boyle PK, Reinhard JP (2015b) Carbon monoxide and iron modulate plasmatic coagulation in Alzheimer’s disease. Curr Neurovasc Res 12:31–39
Orino K (2013) Functional binding analysis of human fibrinogen as an iron- and heme-binding protein. Biometals 26:789–794
Pretorius E, Vermeulen N, Bester J, Lipinski B (2013) Novel use of scanning electron microscopy for detection of iron-induced morphological changes in human blood. Microsc Res Tech 76:268–271
Shah N, Welsby IJ, Fielder MA, Jacobsen WK, Nielsen VG (2015) Sickle cell disease is associated with iron mediated hypercoagulability. J Thromb Thrombolysis 40:182–185
Swanepoel AC, Lindeque BG, Swart PJ, Abdool Z, Pretorius E (2014) Estrogen causes ultrastructural changes of fibrin networks during the menstrual cycle: a qualitative investigation. Microsc Res Tech 77:594–601
Acknowledgements
This investigation was supported by the Department of Anesthesiology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nielsen, V.G., Jacobsen, W.K. Iron modulates the alpha chain of fibrinogen. Biometals 29, 235–238 (2016). https://doi.org/10.1007/s10534-016-9909-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-016-9909-5