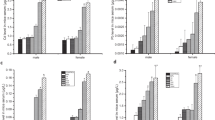
Abstract
Iron is reported to interact with other metals. In addition, it has been shown that genetic background may impact iron metabolism. Our objective was to characterize, in mice of three genetic backgrounds, the links between iron and several non-iron metals. Thirty normal mice (C57BL/6, Balb/c and DBA/2; n = 10 for each group), fed with the same diet, were studied. Quantification of iron, zinc, cobalt, copper, manganese, magnesium and rubidium was performed by ICP/MS in plasma, erythrocytes, liver and spleen. Transferrin saturation was determined. Hepatic hepcidin1 mRNA level was evaluated by quantitative RT-PCR. As previously reported, iron parameters were modulated by genetic background with significantly higher values for plasma iron parameters and liver iron concentration in DBA/2 and Balb/c strains. Hepatic hepcidin1 mRNA level was lower in DBA/2 mice. No iron parameter was correlated with hepcidin1 mRNA levels. Principal component analysis of the data obtained for non-iron metals indicated that metals parameters stratified the mice according to their genetic background. Plasma and tissue metals parameters that are dependent or independent of genetic background were identified. Moreover, relationships were found between plasma and tissue content of iron and some other metals parameters. Our data: (i) confirms the impact of the genetic background on iron parameters, (ii) shows that genetic background may also play a role in the metabolism of non-iron metals, (iii) identifies links between iron and other metals parameters which may have implications in the understanding and, potentially, the modulation of iron metabolism.


Similar content being viewed by others
References
An P et al (2012) TMPRSS6, but not TF, TFR2 or BMP2 variants are associated with increased risk of iron-deficiency anemia. Hum Mol Genet 21:2124–2131. doi:10.1093/hmg/dds028
Andrews NC (1999) Disorders of iron metabolism. N Engl J Med (published erratum appears in N Engl J Med 2000 Feb 3;342(5):364) 341:1986–1995
Balesaria S, Ramesh B, McArdle H, Bayele HK, Srai SK (2010) Divalent metal-dependent regulation of hepcidin expression by MTF-1. FEBS Lett 584:719–725. doi:10.1016/j.febslet.2009.12.023
Bentley SA, Miller DT (1977) Radionucleide blood survival studies. In: Lewis SM (ed) Radioisotopes in haematology. Sauders, London, pp 245–262
Brissot P, Troadec MB, Bardou-Jacquet E, Le Lan C, Jouanolle AM, Deugnier Y, Loreal O (2008) Current approach to hemochromatosis. Blood Rev 22:195–210. doi:10.1016/j.blre.2008.03.001
Butterworth RF (2013) The liver-brain axis in liver failure: neuroinflammation and encephalopathy. Nat Rev Gastroenterol Hepatol 10:522–528. doi:10.1038/nrgastro.2013.99
Camaschella C et al (2000) The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet 25:14–15
Carroll BJ, Sharp PT (1971) Rubidium and lithium: opposite effects on amine-mediated excitement. Science 172:1355–1357
Champy MF et al (2008) Genetic background determines metabolic phenotypes in the mouse. Mamm Genome 19:318–331. doi:10.1007/s00335-008-9107-z
Coppin H et al (2007) Gene expression profiling of Hfe−/− liver and duodenum in mouse strains with differing susceptibilities to iron loading: identification of transcriptional regulatory targets of Hfe and potential hemochromatosis modifiers. Genome Biol 8:R221. doi:10.1186/gb-2007-8-10-r221
Cotzias GC (1958) Manganese in health and disease. Physiol Rev 38:503–532
Courselaud B et al (2004) Strain and gender modulate hepatic hepcidin 1 and 2 mRNA expression in mice. Blood Cells Mol Dis 32:283–289. doi:10.1016/j.bcmd.2003.11.003
Dupic F et al (2002) Inactivation of the hemochromatosis gene differentially regulates duodenal expression of iron-related mRNAs between mouse strains. Gastroenterology 122:745–751
Fawcett WJ, Haxby EJ, Male DA (1999) Magnesium: physiology and pharmacology. Br J Anaesth 83:302–320
Finberg KE et al (2008) Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet 40:569–571
Finberg KE, Whittlesey RL, Andrews NC (2011) Tmprss6 is a genetic modifier of the Hfe-hemochromatosis phenotype in mice. Blood 117:4590–4599. doi:10.1182/blood-2010-10-315507
Garcia SJ, Gellein K, Syversen T, Aschner M (2007) Iron deficient and manganese supplemented diets alter metals and transporters in the developing rat brain. Toxicol Sci 95:205–214. doi:10.1093/toxsci/kfl139
Gunshin H et al (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388:482–488
Hansen SL, Trakooljul N, Liu HC, Hicks JA, Ashwell MS, Spears JW (2010) Proteins involved in iron metabolism in beef cattle are affected by copper deficiency in combination with high dietary manganese, but not by copper deficiency alone. J Anim Sci 88:275–283. doi:10.2527/jas.2009-1846
Harris ZL, Takahashi Y, Miyajima H, Serizawa M, MacGillivray RT, Gitlin JD (1995) Aceruloplasminemia: molecular characterization of this disorder of iron metabolism. Proc Natl Acad Sci USA 92:2539–2543
Island ML, Jouanolle AM, Mosser A, Deugnier Y, David V, Brissot P, Loreal O (2009) A new mutation in the hepcidin promoter impairs its BMP response and contributes to a severe phenotype in HFE related hemochromatosis. Haematologica 94:720–724. doi:10.3324/haematol.2008.001784
Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T (2014) Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet 46:678–684. doi:10.1038/ng.2996
Kim J, Buckett PD, Wessling-Resnick M (2013) Absorption of manganese and iron in a mouse model of hemochromatosis. PLoS ONE 8:e64944. doi:10.1371/journal.pone.0064944
Latour C et al (2014) Testosterone perturbs systemic iron balance through activation of epidermal growth factor receptor signaling in the liver and repression of hepcidin. Hepatology 59:683–694. doi:10.1002/hep.26648
Leboeuf RC, Tolson D, Heinecke JW (1995) Dissociation between tissue iron concentrations and transferrin saturation among inbred mouse strains. J Lab Clin Med 126(2):128–136
Le Gac G et al (2004) The recently identified type 2A juvenile haemochromatosis gene (HJV), a second candidate modifier of the C282Y homozygous phenotype. Hum Mol Genet 13:1913–1918
Loreal O et al (2002) Aceruloplasminemia: new clinical, pathophysiological and therapeutic insights. J Hepatol 36:851–856
Loreal O, Cavey T, Bardou-Jacquet E, Guggenbuhl P, Ropert M, Brissot P (2014) Iron, hepcidin, and the metal connection. Front Pharmacol 5:128. doi:10.3389/fphar.2014.00128
Macmillan-Crow LA, Cruthirds DL (2001) Invited review: manganese superoxide dismutase in disease. Free Radic Res 34:325–336
Mc Ardle BA, Dowsley TF, deKemp RA, Wells GA, Beanlands RS (2012) Does rubidium-82 PET have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease?: A systematic review and meta-analysis. J Am Coll Cardiol 60:1828–1837. doi:10.1016/j.jacc.2012.07.038
Melis MA et al (2008) A mutation in the TMPRSS6 gene, encoding a transmembrane serine protease that suppresses hepcidin production, in familial iron deficiency anemia refractory to oral iron. Haematologica 93:1473–1479
Meltzer HL (1991) A pharmacokinetic analysis of long-term administration of rubidium chloride. J Clin Pharmacol 31:179–184
Merryweather-Clarke AT et al (2003) Digenic inheritance of mutations in HAMP and HFE results in different types of haemochromatosis. Hum Mol Genet 12:2241–2247
Milet J et al (2007) Common variants in the BMP2, BMP4, and HJV genes of the hepcidin regulation pathway modulate HFE hemochromatosis penetrance. Am J Hum Genet 81:799–807. doi:10.1086/520001
Mitchell CJ, Shawki A, Ganz T, Nemeth E, Mackenzie B (2014) Functional properties of human ferroportin, a cellular iron exporter reactive also with cobalt and zinc. Am J Physiol Cell Physiol 306:C450–C459. doi:10.1152/ajpcell.00348.2013
Mukhopadhyay CK, Attieh ZK, Fox PL (1998) Role of ceruloplasmin in cellular iron uptake. Science 279:714–717
Nai A et al (2011) TMPRSS6 rs855791 modulates hepcidin transcription in vitro and serum hepcidin levels in normal individuals. Blood 118:4459–4462. doi:10.1182/blood-2011-06-364034
Olatunbosun D, Corbett WE, Ludwig J, Valberg LS (1970) Alteration of cobalt absorption in portal cirrhosis and idiopathic hemochromatosis. J Lab Clin Med 75:754–762
Papanikolaou G et al (2004) Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet 36:77–82. doi:10.1038/ng1274ng1274
Papavasiliou PS, Miller ST, Cotzias GC (1966) Role of liver in regulating distribution and excretion of manganese. Am J Physiol 211:211–216
Pollack S, George JN, Reba RC, Kaufman RM, Crosby WH (1965) The absorption of nonferrous metals in iron deficiency. J Clin Invest 44:1470–1473. doi:10.1172/JCI105253
Rempoulakis P et al (2014) Conserved metallomics in two insect families evolving separately for a hundred million years. Biometals 27:1323–1335. doi:10.1007/s10534-014-9793-9
Roetto A et al (2003) Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet 33:21–22
Sadraie M, Missirlis F (2011) Evidence for evolutionary constraints in Drosophila metal biology. Biometals 24:679–686. doi:10.1007/s10534-011-9420-y
Siegers MP, Kasperek K, Heiniger HJ, Feinendegen LE (1977) Distribution of trace elements in organs of mice of different inbred strains. J Radioanal Chem 37:421–426
Simonsen LO, Harbak H, Bennekou P (2012) Cobalt metabolism and toxicology: a brief update. Sci Total Environ 432:210–215. doi:10.1016/j.scitotenv.2012.06.009
Troadec MB, Ward DM, Lo E, Kaplan J, De Domenico I (2010) Induction of FPN1 transcription by MTF-1 reveals a role for ferroportin in transition metal efflux. Blood 116:4657–4664. doi:10.1182/blood-2010-04-278614
Tuoni M et al (1987) Renal tolerance of rubidium chloride: short-term clinical evaluation. J Clin Pharmacol 27:503–507
Videt-Gibou D et al (2009) Iron excess treatable by copper supplementation in acquired aceruloplasminemia: a new form of secondary human iron overload? Blood 114:2360–2361. doi:10.1182/blood-2009-06-226175
Wang F et al (2007) Genetic variation in Mon1a affects protein trafficking and modifies macrophage iron loading in mice. Nat Genet 39(8):1025–1032
Weiss G, Goodnough LT (2005) Anemia of chronic disease. N Engl J Med 352:1011–1023
Yamaguchi S et al (2007) Effects of lead, molybdenum, rubidium, arsenic and organochlorines on spermatogenesis in fish: monitoring at Mekong Delta area and in vitro experiment. Aquat Toxicol 83:43–51. doi:10.1016/j.aquatox.2007.03.010
Acknowledgments
This work was supported by INSERM and University of Rennes 1.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cavey, T., Ropert, M., de Tayrac, M. et al. Mouse genetic background impacts both on iron and non-iron metals parameters and on their relationships. Biometals 28, 733–743 (2015). https://doi.org/10.1007/s10534-015-9862-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-015-9862-8