Abstract
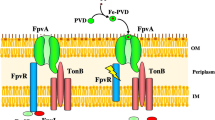
The bacterial siderophore pyochelin is composed of salicylate and two cysteine-derived heterocycles, the second of which is modified by reduction and N-methylation during biosynthesis. In Pseudomonas aeruginosa, the first cysteine residue is converted to its D-isoform during thiazoline ring formation, whereas the second cysteine remains in its L-configuration. Stereochemistry is opposite in the Pseudomonas fluorescens siderophore enantio-pyochelin, in which the first ring originates from L-cysteine and the second ring from D-cysteine. Both siderophores promote growth of the producer organism during iron limitation and induce the expression of their biosynthesis genes by activating the transcriptional AraC-type regulator PchR. However, neither siderophore is functional as an iron carrier or as a transcriptional inducer in the other species, demonstrating that both processes are highly stereospecific. Stereospecificity of pyochelin/enantio-pyochelin-mediated iron uptake is ensured at two levels: (i) by the outer membrane siderophore receptors and (ii) by the cytosolic PchR regulators.





Similar content being viewed by others
References
Andrews SC, Robinson AK, Rodríguez-Quiñones F (2003) Bacterial iron homeostasis. FEMS Microbiol Rev 27:215–237
Ankenbauer RG, Quan HN (1994) FptA, the Fe(III)-pyochelin receptor of Pseudomonas aeruginosa: a phenolate siderophore receptor homologous to hydroxamate siderophore receptors. J Bacteriol 176:307–319
Ankenbauer RG, Toyokuni T, Staley A, Rinehart KL Jr, Cox CD (1988) Synthesis and biological activity of pyochelin, a siderophore of Pseudomonas aeruginosa. J Bacteriol 170:5344–5351
Bodilis J, Cornelis P (2009) A survey of TonB-dependent receptors in fluorescent pseudomonads. Environ Microbiol Rep 1:256–262
Braud A, Hannauer M, Mislin GLA, Schalk IJ (2009) The Pseudomonas aeruginosa pyochelin-iron uptake pathway and its metal specificity. J Bacteriol 191:3517–3525
Budzikiewicz H (2004) Siderophores of the Pseudomonadaceae sensu stricto (fluorescent and non-fluorescent Pseudomonas spp.). Fortschr Chem Org Naturst 87:81–237
Castignetti D (1997) Probing of Pseudomonas aeruginosa, Pseudomonas aureofaciens, Burkholderia (Pseudomonas) cepacia, Pseudomonas fluorescens, and Pseudomonas putida with the ferripyochelin receptor A gene and the synthesis of pyochelin in Pseudomonas aureofaciens, Pseudomonas fluorescens, and Pseudomonas putida. Curr Microbiol 34:250–257
Cobessi D, Celia H, Pattus F (2005) Crystal structure at high resolution of ferric-pyochelin and its membrane receptor FptA from Pseudomonas aeruginosa. J Mol Biol 352:893–904
Cornelis P (2010) Iron uptake and metabolism in pseudomonads. Appl Microbiol Biotechnol 86:1637–1645
Cox CD (1980) Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J Bacteriol 142:581–587
Cox CD, Graham R (1979) Isolation of an iron-binding compound from Pseudomonas aeruginosa. J Bacteriol 137:357–364
Cox CD, Rinehart KL, Moore ML, Cook JC (1981) Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc Natl Acad Sci USA 78:4256–4260
DeClue MS, Baldridge KK, Künzler DE, Kast P, Hilvert D (2005) Isochorismate pyruvate lyase: a pericyclic reaction mechanism? J Am Chem Soc 127:15002–15003
Drechsel H, Jung G, Winkelmann G (1992) Stereochemical characterization of rhizoferrin and identification of its dehydration products. Biometals 5:141–148
Escolar L, Perez-Martin J, de Lorenzo V (1999) Opening the iron box: transcriptional metalloregulation by the Fur protein. J Bacteriol 181:6223–6229
Gaille C, Kast P, Haas D (2002) Salicylate biosynthesis in Pseudomonas aeruginosa. J Biol Chem 277:21768–21775
Gaille C, Reimmann C, Haas D (2003) Isochorismate synthase (PchA), the first and rate-limiting enzyme in salicylate biosynthesis of Pseudomonas aeruginosa. J Biol Chem 278:16893–16898
Guerinot ML (1994) Microbioal iron transport. Annu Rev Microbiol 48:743–772
Harrison AJ, Yu M, Gårdenborg T, Middleditch M, Ramsay RJ, Baker EN, Lott JS (2006) The structure of MbtI from Mycobacterium tuberculosis, the first enzyme in the biosynthesis of the siderophore mycobactin, reveals it to be a salicylate synthase. J Bacteriol 188:6081–6091
Hayen H, Volmer DA (2006) Different iron-chelating properties of pyochelin diastereoisomers revealed by LC/MS. Anal Bioanal Chem 385:606–611
Heinrichs DE, Poole K (1993) Cloning and sequence analysis of a gene (pchR) encoding an AraC family activator of pyochelin and ferripyochelin receptor synthesis in Pseudomonas aeruginosa. J Bacteriol 175:5882–5889
Heinrichs DE, Poole K (1996) PchR, a regulator of ferripyochelin receptor gene (fptA) expression in Pseudomonas aeruginosa, functions both as an activator and a repressor. J Bacteriol 178:2586–2592
Hoegy F, Lee X, Noel S, Rognan D, Mislin GL, Reimmann C, Schalk IJ (2009) Stereospecificity of the siderophore pyochelin outer membrane transporters in fluorescent pseudomonads. J Biol Chem 284:14949–14957
Ino A, Murabayashi A (2001) Synthetic studies of thiazoline and thiazolidine-containing natural products. Part 3: total synthesis and absolute configuration of the siderophore yersiniabactin. Tetrahedron 57:1897–1902
Kerbarh O, Ciulli A, Howard NI, Abell C (2005) Salicylate biosynthesis: overexpression, purification, and characterization of Irp9, a bifunctional salicylate synthase from Yersinia enterocolitica. J Bacteriol 187:5061–5066
Kerbarh O, Chirgadze DY, Blundell TL, Abell C (2006) Crystal structures of Yersinia enterocolitica salicylate synthase and its complex with the reaction products salicylate and pyruvate. J Mol Biol 357:524–534
Klumpp C, Burger A, Mislin GL, Abdallah MA (2005) From a total synthesis of cepabactin and its 3:1 ferric complex to the isolation of a 1:1:1 mixed complex between iron(III), cepabactin and pyochelin. Bioorg Med Chem Lett 15:1721–1724
Köster W (2001) ABC transporter-mediated uptake of iron, siderophores, heme and vitamin B12. Res Microbiol 152:291–301
Künzler DE, Sasso S, Gamper M, Hilvert D, Kast P (2005) Mechanistic insights into the isochorismate pyruvate-lyase activity of the catalytically promiscuous PchB from combinatorial mutagenesis and selection. J Biol Chem 280:32827–32834
Liu PV, Shokrani F (1978) Biological activities of pyochelins: iron-chelating agents of Pseudomonas aeruginosa. Infect Immun 22:878–890
Meyer J-M (2000) Pyoverdines: pigments, siderophores and potential taxonomic markers of fluorescent Pseudomonas species. Arch Microbiol 174:135–142
Michel L, Gonzalez N, Jagdeep S, Nguyen-Ngoc T, Reimmann C (2005) PchR-box recognition by the AraC-type regulator PchR of Pseudomonas aeruginosa requires the siderophore pyochelin as an effector. Mol Microbiol 58:495–509
Michel L, Bachelard A, Reimmann C (2007) Ferripyochelin uptake genes are involved in pyochelin-mediated signalling in Pseudomonas aeruginosa. Microbiology 153:1508–1518
Münzinger M, Taraz K, Budzikiewicz H, Drechsel H, Heymann P, Winkelmann G, Meyer J-M (1999) S,S-rhizoferrin (enantio-rhizoferrin)—a siderophore of Ralstonia (Pseudomonas) pickettii DSM 6297—the optical antipode of R,R-rhizoferrin isolated from fungi. Biometals 12:189–193
Namiranian S, Richardson DJ, Russell DA, Sodeau JR (1997) Excited state properties of the siderophore pyochelin and its complex with zinc ions. Photochem Photobiol 65:777–782
Ó Cuív P, Clarke P, Lynch D, O’Connell M (2004) Identification of rhtX and fptX, novel genes encoding proteins that show homology and function in the utilization of the siderophores rhizobactin 1021 by Sinorhizobium meliloti and pyochelin by Pseudomonas aeruginosa, respectively. J Bacteriol 186:2996–3005
Ochsner UA, Vasil AI, Vasil ML (1995) Role of the ferric uptake regulator of Pseudomonas aeruginosa in the regulation of siderophores and exotoxin A expression: purification and activity on iron-regulated promoters. J Bacteriol 177:7194–7201
Patel HM, Walsh CT (2001) In vitro reconstitution of the Pseudomonas aeruginosa nonribosomal peptide synthesis of pyochelin: characterization of backbone tailoring thiazoline reductase and N-methyltransferase activities. Biochemistry 40:9023–9031
Patel HM, Tao J, Walsh CT (2003) Epimerization of an L-cysteinyl to a D-cysteinyl residue during thiazoline ring formation in siderophore chain elongation by pyochelin synthetase from Pseudomonas aeruginosa. Biochemistry 42:10514–10527
Paulsen IT, Press CM, Ravel J, Kobayashi DY, Myers GSA et al (2005) Complete genome sequence of the plant commensal Pseudomonas fluorescens Pf-5. Nat Biotechnol 23:873–878
Pelludat C, Brem D, Heesemann J (2003) Irp9, encoded by the high-pathogenicity island of Yersinia enterocolitica, is able to convert chorismate into salicylate, the precursor of the siderophore yersiniabactin. J Bacteriol 185:5648–5653
Quadri LEN, Keating TA, Patel HM, Walsh CT (1999) Assembly of the Pseudomonas aeruginosa nonribosomal peptide siderophore pyochelin: in vitro reconstitution of aryl-4,2-bisthiazoline synthetase activity from PchD, PchE and PchF. Biochemistry 38:14941–14954
Reimmann C, Serino L, Beyeler M, Haas D (1998) Dihydroaeruginoic acid synthetase and pyochelin synthetase, products of the pchEF genes are induced by extracellular pyochelin in Pseudomonas aeruginosa. Microbiology 144:3135–3148
Reimmann C, Patel HM, Serino L, Barone M, Walsh CT, Haas D (2001) Essential PchG-dependent reduction in pyochelin biosynthesis of Pseudomonas aeruginosa. J Bacteriol 183:813–820
Reimmann C, Patel HM, Walsh CT, Haas D (2004) PchC thioesterase optimizes nonribosomal biosynthesis of the peptide siderophore pyochelin in Pseudomonas aeruginosa. J Bacteriol 186:6367–6373
Rinehart KL, Staley AL, Wilson SR, Ankenbauer RG, Cox CD (1995) Stereochemical assignment of the pyochelins. J Org Chem 60:2786–2791
Schlegel K, Taraz K, Budzikiewicz H (2004) The stereoisomers of pyochelin, a siderophore of Pseudomonas aeruginosa. Biometals 17:409–414
Schlegel K, Lex J, Taraz K, Budzikiewicz (2006) The X-ray structure of the pyochelin Fe3+ complex. Z Naturforsch 61c:263–266
Schmidli-Sacherer P, Keel C, Défago G (1997) The global regulator GacA of Pseudomonas fluorescens CHA0 is required for suppression of root diseases in dicotyledons but not in Gramineae. Plant Pathol 46:80–90
Serino L, Reimmann C, Baur H, Beyeler M, Visca P, Haas D (1995) Structural genes for salicylate biosynthesis from chorismate in Pseudomonas aeruginosa. Mol Gen Genet 249:217–228
Serino L, Reimmann C, Visca P, Beyeler M, Della Chiesa V, Haas D (1997) Biosynthesis of pyochelin and dihydroaeruginoic acid requires the iron-regulated pchDCBA operon in Pseudomonas aeruginosa. J Bacteriol 179:248–257
Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P et al (2000) Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964
Terano H, Nomoto K, Takase S (2002) Siderophore production and induction of iron-regulated proteins by a microorganism from rhizosphere of barley. Biosci Biotechnol Biochem 66:2471–2473
Thieken A, Winkelmann G (1992) Rhizoferrin: a complexone type siderophore of the Mucorales and Entomophthorales (Zygomycetes). FEMS Microbiol Lett 94:37–42
Thomas MS (2007) Iron acquisition mechanisms of the Burkholderia cepacia complex. Biometals 20:431–452
Tseng C-F, Burger A, Mislin GLA, Schalk IJ, Yu SS-F, Cahn SI, Abdallah MA (2006) Bacterial siderophores: the solution stoichiometry and coordination of the Fe(III) complexes of pyochelin and related compounds. J Biol Inorg Chem 11:419–432
Visca P, Colotti G, Serino L, Verzili D, Orsi N, Chiancone E (1992) Metal regulation of siderophore synthesis in Pseudomonas aeruginosa and functional effects of siderophore-metal complexes. Appl Environ Microbiol 58:2886–2893
Wandersman C, Delepelaire P (2004) Bacterial iron sources: from siderophores to hemophores. Annu Rev Microbiol 58:611–647
Youard ZA, Reimmann C (2010) Stereospecific recognition of pyochelin and enantio-pyochelin by the PchR proteins in fluorescent pseudomonads. Microbiology 156:1772–1782
Youard ZA, Mislin GLA, Majcherczyk PA, Schalk IJ, Reimmann C (2007) Pseudomonas fluorescens CHA0 produces enantio-pyochelin, the optical antipode of the Pseudomonas aeruginosa siderophore pyochelin. J Biol Chem 282:35546–35553
Zaitseva J, Lu J, Olechoski KL, Lamb AL (2006) Two crystal structures of the isochorismate pyruvate-lyase from Pseudomonas aeruginosa. J Biol Chem 281:33441–33449
Acknowledgments
We wish to thank Dieter Haas for critical reading of the manuscript. This work was supported by the Swiss National Science Foundation for Scientific Research (Project 31-113955/1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Youard, Z.A., Wenner, N. & Reimmann, C. Iron acquisition with the natural siderophore enantiomers pyochelin and enantio-pyochelin in Pseudomonas species. Biometals 24, 513–522 (2011). https://doi.org/10.1007/s10534-010-9399-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-010-9399-9