Abstract
Microbially-mediated iron (Fe) cycling controls the fate of organic matter, contaminants, and nutrients in terrestrial ecosystems including wetland soils. However, the effects of temperature variations due to seasonal differences on Fe(III) reduction rates and kinetics in such ecosystems remains poorly understood. To evaluate the potential temperature impact on dissimilatory microbial Fe(III) reduction it is crucial to determine environmentally-relevant reaction rates and kinetic parameters. Here, we investigate the relationship between soil temperature and microbial Fe(III) reduction kinetics in mineral soils from two subalpine wetlands with distinct hydrologic and edaphic conditions. We conducted flow-through experiments (FTR) at three temperatures (6, 12, and 18 °C) using intact soil cores collected from 30 cm [(higher organic carbon (Corg) and total nitrogen (TN)] and 70 cm (lower Corg and TN) soil depths in order to determine the apparent Fe(III) affinity constant (Km), apparent maximum Fe(III) reduction rates (Vmax) and temperature sensitivity (Q10 and Ea) of Fe(III) reduction. We used Fe(III)-NTA, a model compound for aqueous labile and complexed Fe present in natural organic matter. Our results show that changes in apparent Vmax and Km are driven primarily by temperature. Significant differences in apparent Vmax at 18 °C relative to 6 and 12 °C (P < 0.05) suggest that dissimilatory microbial Fe(III) reduction in wetland soils accelerates during warmer summer days. However, temperature alone fails to explain the large variability of the apparent parameters Q10 (1.5–8.9) and Ea (26–148 kJ mol−1) for the two wetland types and depths (30 and 70 cm). Strong relationship between both parameters of temperature sensitivity (Q10 and Ea) and reactive soil Fe content at 30 cm and Corg/TN at 70 cm depth demonstrate the notable impact of soil properties on the temperature sensitivity for mirobial Fe(III) reduction in these wetland soils. Our results emphasize the importance of soil temperature on Fe(III) reduction kinetics and must be considered when predicting dissimilatory Fe(III) reduction under different seasonal temperatures or in wetlands located at different temperature regimes.
Similar content being viewed by others
Introduction
Iron (Fe) is one of the most abundant redox-sensitive metals, which influences water quality by buffering redox conditions, and regulating storage and release of carbon (e.g., Wagai and Mayer 2007; Amstaetter et al. 2012; Shimizu et al. 2013; Pan et al. 2016; Zhao et al. 2016; Hall et al. 2018) nutrients (e.g., Wilson et al. 2004; Morton et al. 2005), and contaminants (e.g., Zachara et al. 2001; Borch et al. 2010) in terrestrial environments. Wetlands specifically are ‘biogeochemical hot spots’ of Fe cycling (Roden and Wetzel 2002; Johnston et al. 2014). Wetland Fe cycling is dominated by precipitation and immobilization of Fe(III) under oxic conditions and by mobilization of Fe(II) via reductive dissolution under reducing conditions (e.g., Lovley and Phillips 1991; Roden and Wetzel 2002; Roden 2003; Lovley et al. 2004; Baldwin et al. 2006; Riedel et al. 2013). Both dissimilatory microbial Fe(III) reduction and abiotic reductive dissolution of Fe(III)-oxyhydroxides (e.g., goethite, lepidocrocite, ferrihydrite) can lead to the release of organic carbon (Corg) bound to oxide minerals (Lovley and Phillips 1991; Wagai and Mayer 2007; Lalonde et al. 2012; Pan et al. 2016, Zhao et al. 2016). Dissimilatory microbial Fe(III) reduction has also been shown to inhibit methane (CH4) production in Fe(III)-rich environments (Kruger et al. 2001; Kogel-Knabner et al. 2010; Herndon et al. 2015; Yang et al. 2016).
Although it is recognized that microbial processes are temperature-dependent (e.g., Buford Price and Sowers 2004; Schipper et al. 2014; Bååth 2018), the role of seasonal temperature fluctuations on Fe(III) reduction rates and kinetics remains poorly understood. Specially, high elevation wetlands might be particularly sensitive to seasonal temperature variation as soil microbes from high altitude and latitude ecosystems are extremely sensitive to temperature increase (Belotte et al. 2003; Davidson et al. 2006; German et al. 2012; Blagodatskaya et al. 2015). Indeed, enzymatic activities of soil microbes are adapted to the prevailing temperature of their habitats (Belotte et al. 2003; German et al. 2012) and enzymes adapted to colder climate tend to be more sensitive to increasing temperature than enzymes from warmer climates (Siddiqui and Cavicchioli 2006; Dong and Somero 2009; German et al. 2012). Therefore, a better mechanistic understanding of the temperature sensitivity of microbial Fe(III) reduction kinetics is required to predict seasonal variations in Fe(III) reduction and cycling.
Our current knowledge of Fe(III) reduction kinetics is largely based on simplified laboratory conditions using pure bacterial cultures and slurry experiments (Liu et al. 2002; Bonneville et al. 2004; Hyacinthe et al. 2006). These studies, however, largely neglect the effects of temperature and ignore the physical, geochemical, and microbiological complexity of soils. By eliminating mass transfer limitations (e.g., diffusion, dispersion, advection), conventional slurry experiments result in an artificially high delivery of substrates to microorganisms and thus enhance the reaction rates. Thus, there is a critical need for verification of these studies in biogeochemically complex, natural environments.
Our objectives are to (1) determine the impact of average ‘seasonal’ temperature variation on Fe(III) reduction kinetics in two distinct wetland types and (2) to evaluate how physical, chemical, and microbial soil properties control microbial Fe(III) reduction rates as temperature changes. We hypothesize that the kinetics of microbial Fe(III) reduction (Vmax and Km) change with increasing temperature, and that differences in soil properties between two contrasting wetland types (depressional and slope wetland) affect the temperature sensitivity (Q10 and Ea). Additionally, spatial differences in soil properties within these wetlands such as Fe(III) concentration and organic matter content (Roden and Wetzel 2002; Wagai and Mayer 2007; Lalonde et al. 2012; Zhao et al. 2016) can shape the native soil microbial community and can result in high variability in Fe(III) reduction potential (Weiss et al. 2004). We conducted flow-through reactor (FTR) experiments using undisturbed wetland soils cores allowing a direct comparison of Fe(III) reduction rates at different temperature settings while (1) preserving the soil porosity, (2) the solution to solid ratio, and (3) the spatial distribution of the microorganisms.
Materials and methods
Site description and sampling
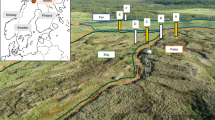
The depressional and slope-type wetlands (Carsey et al. 2003) are located in the subalpine forest (2700-3300 m elevation) in the USFS Fraser Experimental Forest (Rocky Mountains, Colorado, USA), (Fig. 1). The subalpine forest region has a mean annual air temperature of 0.6 °C with monthly means ranging from about − 10 °C in January to 12.2 °C in July (Fig. 1a; Elder 2006; LaPerriere et al. 2011). Formed within topographic depressions, the depressional wetland is inundated annually by spring snowmelt and then dries over the course of two months. The depressional wetland is characterized by long water residence times ranging from weeks to months (Fig. 1c) and high accumulation of organic matter from surrounding uplands. In contrast, slope wetlands are supplied by perennial groundwater springs and maintain a relatively constant water table (Fig. 1d). The constant drainage of the slope wetland leads to short water residence time (hours to days), constant redox conditions throughout the year and throughout the wetland (Brinson 1993) and limits the accumulation of dissolved and particulate organic substrates and nutrients.
a Average monthly air temperature from 1976 to 2003 at the USFS Fraser Experimental Forest derived from hourly temperature measurements (Elder 2006). Error bars show standard deviations over the period 1976–2003. Red dashed lines denote the temperatures used for the flow-through experiments (FTR). b Wetland locations at the USFS Fraser Experimental Forest in the Rocky Mountains, Colorado, USA. Schematics of c depressional and d slope wetland. For the depressional wetland, soil cores (blue column = waterlogged; brown dashed column = fluctuating water table) were collected across a hydroperiod and redox gradient. For the slope wetland, a soil core (blue column = waterlogged) was sampled at the base of the wetland
For the depressional wetland, we selected three sampling locations across a redox gradient from the permanently waterlogged center, to the seasonally-waterlogged transition and temporarily waterlogged wetland edge (Fig. 1c). For the slope wetland, we sampled a single location near the base of the wetland (Fig. 1d). At all locations, we collected duplicate soil cores (100 cm-long, 6 cm diameter). For all sampled soil cores, we discarded the O horizon (0–30 cm) and used only the mineral soil horizon from which we extracted 2 cm-long soil slices from 30 and 70 cm depths. These two depths were chosen to compare changes in physical and chemical soil properties within each location. Both physico-chemical soil characterization and FTR were performed on soil samples of the same depth and core. All soil samples were stored under anoxic conditions at 4 °C until experiments or soil analyses were performed.
Physico-chemical and microbial soil characterization
Soil pH, bulk density, porosity, organic C (Corg) content, total N (TN) content and extractable reactive Fe content were determined on duplicate subsamples from the two soil depths. The soil pH was measured in 0.1 M CaCl2 at a soil/solution ratio of 1:20 (Fullen and Catt 2014). Soil dry bulk density (ρd in g cm−3) was determined after drying a known soil volume for 24 h at 105 °C (Blake and Hartge 1986). Soil porosity (%) was calculated from the dry bulk density and particle density (ρs = 2.65 g cm−3) as 1-(ρd/ρS). Soil Corg and TN (wt %) were measured on air-dried samples (Nelson and Sommers 1986) using a dry combustion CN analyzer (Carlo Erba Elantech elemental analyzer, Lakewood, NJ, USA). Soil reactive Fe content was extracted by digesting 2 g of wet soil in 30 mL of 1 M HCl for 24 h (e.g., Slomp et al. 1997; Roden and Wetzel 2002; Hyacinthe et al. 2006) and determined spectrophotometrically (wavelength 562 nm) by ferrozine with hydroxylamine (Stookey 1970). The Fe extraction with HCl intends to assess reactive Fe(III) soil phases (Hyacinthe et al. 2006; Roden and Wetzel 2002), which are the primary terminal electron acceptors and thus most impacted by microbial Fe(III) reduction.
Culturable Fe(III)-reducing bacteria were enumerated by the most-probable-number (MPN) technique (Woomer 1994). Five grams of fresh bulk soil were suspended in 50 mL sterile saline solution (2% NaCl; 0.3% MgCl2) and shaken thoroughly. Tenfold triplicate serial dilutions of those soil slurries were made directly in the culture medium. The medium was similar to the synthetic porewater used in the FTR experiments (as described below) and was supplemented with 1 mL of trace metal solution, 1 mL selenium-tungsten solution and 1 mL of 1% yeast extract (Supplementary Table S1, adapted from Sanford et al. 2007). After autoclaving we added Fe(III) complexed with nitrilotriacetic acid [Fe(III)-NTA] as electron acceptor (final concentration of 10 mM), and both lactate and acetate (final concentration of 20 mM each) as electron donors from sterile anoxic stock solutions. The serial cultures were incubated at 30 °C for 4 weeks. The presence of Fe(III)-reducing bacteria was scored positive upon the presence of Fe(II), tested using the ferrozine assay (Stookey 1970; Lovley and Woodward 1996).
Flow-through reactor experiments
Flow-through reactor (FTR) experiments were performed to measure the potential rates of Fe(III) reduction (Fe(III)RR) and Fe(II) export (Fe(II)ER), which represent the fraction of Fe(II) exported from the soil to the FTR output solution. Such FTR experiments have been previously used to determine potential reaction rates and apparent kinetic parameters for sulfate reduction (Roychoudhury et al. 1998; Pallud and Van Cappellen 2006; Pallud et al. 2007; Stam et al. 2010, 2011; Tarpgaard et al. 2011; Richards and Pallud 2016), nitrate reduction (Laverman et al. 2006; 2012; Gu et al. 2012), and selenate reduction (Schilling et al. 2018). The advantages of the FTR are that reaction rates are determined for near steady-state conditions and dissolved metabolic byproducts do not accumulate in the reactor (Pallud and Van Cappellen 2006). The reaction rates obtained from our experiments are referred to as “potential” rates, as they correspond to Fe(III) reduction activity when aqueous organically complexed Fe(III) is the primary terminal electron acceptor available for the soil microbial community (Pallud and Van Cappellen 2006). FTR experiments were performed on duplicate cores from two locations (depressional wetland, transition site and slope wetland, both 30 cm depth interval), and considering the extremely good reproducibility of those (see Section “Kinetics of microbial Fe(III) reduction rates”), we conducted only one replicate for the other soils.
Each FTR contained an undisturbed soil core within a Plexiglas ring of 2 cm length and 4.2 cm inside diameter, with 0.2 µm pore size PVDF filters and glass fiber filters at each end. The FTR were sealed with Plexiglas caps kept in place using steel screws while O-rings prevented leakage. Input/output channels opened at the center of the caps at the contact with the glass fibers filters and create a uniform flow throughout the cross-section of the soils (Pallud and Van Cappellen 2006). The experiments were conducted in an anaerobic chamber with a H2/N2 (3–5%/97%) atmosphere and the FTR reactors were placed into a thermostated water bath to ensure constant temperatures of 6, 12 or 18 °C.
An input solution was supplied to the FTRs at a constant volumetric flow rate of 2.0 ± 0.1 mL h−1 (corresponding to total flux of 0.3 L/m2/h) controlled by a peristaltic pump. This flow rate corresponds to water residence times between 8.3 and 12.5 h for most reactors conforming to flow conditions observed in the field for the slope wetland. Each FTR was supplied with four to five successive inflow concentrations for an average of 5 days each to reach steady-state Fe(III) concentrations in the output solution. The experiments at three different temperatures (i.e., 6, 12 and 18 °C) were performed successively. To test temperature sensitivity in a seasonal framework the length of our FTR experiments was on average 30 days for each temperature setting.
The input solution was a synthetic porewater mimicking the major chemical composition of the porewater in the field that contained cations (in mg L−1) of Ca2+ 1.47, Mg2+ 0.32, Na+ 0.41, NH4+ 0.03 and K+ 0.01, all added as either chloride or phosphate salts and the pH was adjusted to 6.15 ± 0.05 to match the average field porewater. The input solutions were purged with O2-free N2. Fe(III) was added to the input solution as Fe(III)-NTA, a model compound for aqueous labile and complexed Fe present in natural organic matter (Lovley and Woodward 1996). The chosen input Fe(III) concentrations ranged between 0.15 and 10 mM to cover previously-reported Km for Fe(III) (Table 3). The high Fe(III) input concentration were chosen to ensure observable differences between Fe(III) input and output concentrations without leading to a complete consumption of Fe(III) and to quantify the maximum temperature sensitivity of Vmax possible for these wetland soils when Fe(III) input is not limited. Over the experimental duration, the input solution was covered with aluminum foil to avoid photochemical NTA degradation (Trott et al. 1972). Output samples were collected for 6 h each and total dissolved Fe and Fe(II) concentrations were determined spectrophotometrically (wavelength 562 nm) by the ferrozine method (Stookey 1970). Dissolved Fe(III) concentrations in the outflow were calculated as the difference between total dissolved Fe and Fe(II) concentrations.
Potential Fe(III) reduction rates (Fe(III)RR) and Fe(II) export rates (Fe(II)ER)
Potential steady-state Fe(III) reduction rates (Fe(III)RRs in nmol cm−3 h−1) were calculated as:
where Fe(III)input is the imposed input Fe(III) concentration (nmol mL−1), Fe(III)output is the steady-state Fe(III) concentration measured in the outflow (nmol mL−1), Q is the volumetric flow rate (mL h−1) and V is the volume of soil in the reactor (cm3) (Pallud et al. 2007). Average Fe(III)RRs were calculated from 14 to 20 data points of steady-state Fe(III) concentrations in the outflow.
Potential steady-state Fe(II) export rates (Fe(II)ERs in nmol cm−3 h−1), which represent the fraction of Fe(II) exported from the soil to the FTR outflow were calculated as:
where Fe(II)output is the steady-state Fe(II) concentration measured in the FTR outflow (nmol mL−1). Average Fe(II)ERs were calculated from 14 to 20 steady-state data points. As Fe(II) is released as complexed and weakly sorbing Fe(II)-NTA (Urrutia et al. 1999), the Fe(II)ERs were expected to scale with Fe(III)RRs.
Determination of apparent kinetic parameters for microbial Fe(III) reduction
The utilization of a substrate by a soil microbial community, or more specifically, the consumption of Fe(III) by Fe(III)-reducing bacteria is widely described by the so-called Michaelis–Menten rate equation (Bonneville et al. 2004; Hyacinthe et al. 2006):
where Vmax is the maximum Fe(III) reduction rate (nmol cm−3 h−1), Km is the affinity, or half-saturation constant for Fe(III) (mM), and Fe(III) is the variable average steady-state Fe(III) concentration. The average steady-state Fe(III) concentration (mM) in the FTR can be approximated as (Fe(III)input + Fe(III)output)/2 (Pallud et al. 2007). The kinetic parameters obtained with from the FTRs are referred to as “apparent” parameters because they reflect the response of the native microbial soil communities under conditions where factors other than the Fe(III) concentration may be limiting (Pallud and Van Cappellen 2006). These apparent kinetic parameters for Fe(III) reduction, Vmax and Km were obtained for each FTR experiment from the average steady-state Fe(III)RRs calculated with Eq. (1), and the average steady-state Fe(III) concentrations by performing a non-linear regression fit of the Michaelis–Menten expression to the data using the KaleidaGraph 4.5.2 software. By omitting other electron acceptors (i.e., nitrate, sulfate) and providing a highly bioavailable Fe(III) form, our kinetic parameters for microbial Fe(III) reduction are at the higher end boundary of what could happen in situ in these wetland soils.
The temperature dependence of Vmax was expressed both as Arrhenius activation energy (Ea) and the temperature coefficient (Q10), which corresponds to the relative increase of the reaction rate for a 10 °C increase in temperature. Using the linear regression coefficient (Coef) from the slope of ln(Vmax) as a function of the absolute temperature 1/T in Kelvin, we determined the apparent Arrhenius activation energy (Ea, kJ mol−1) as:
where R is the universal gas constant.
The apparent Q10 value was calculated as:
where Coef is the regression coefficient obtained from the linear regression of log(Vmax) as a function of temperature (°C) (Atkin et al. 2000).
Statistical analyses
One-way analysis of variance (ANOVA) with Tukey-HSD test (α = 0.05) was used to evaluate the significance of the apparent Fe(III) reduction kinetics (Vmax and Km) at the three temperature settings and for the two soil depths. Two-way ANOVA was used to determine whether soil physico-chemical properties interact with and significantly change the apparent Fe(III) reduction kinetics (Vmax and Km) with increasing temperature. The relationship between soil properties and temperature coefficient Q10 at the two soil depths was examined using the bivariate data fit. All statistical analyses were performed using JMP software 13.1.0.
Results
Selected soil physical, chemical and microbial characteristics
The soils’ bulk density, porosity (P < 0.05), reactive Fe content, Corg, TN and, Corg/TN differed singificantly between the two wetland types (P < 0.01) (Table 1). Soil bulk density and reactive Fe content increased and soil porosity, Corg and TN decreased with soil depth for both wetlands.
The depressional wetland soils had higher Corg and TN than the slope wetland soils (P < 0.05; Table 1). In contrast, reactive Fe(III) content was lower in the depressional than in the slope wetland (P < 0.01; Table 1). The abundance of culturable Fe(III)-reducing bacteria at 30 cm depth ranged from 3.2 to 9.8 × 108 cells cm−3 with no significant differences between the two wetlands or the sampling locations (Table 1). For the 70 cm soil depth, the abundance of Fe(III)-reducing bacteria varied between 4.7 × 107 cells cm−3 for the slope wetland and 1.5 and 1.9 × 109 cells cm−3 for the depressional wetland.
Soil chemical characteristics determined on soil subsamples collected at the end of the FTR experiments (data not shown) show that soil pH (r = 0.76; P < 0.01), soil Corg (r = 0.97; P < 0.01), TN (r = 0.97; P < 0.01) and Corg/TN (r = 0.95, P < 0.01) were not significantly altered during the experiment.
Flow-through reactor experiments
We observed a fast Fe(III) breakthrough after 6 hours (0.5 pore volumes) for all FTR experiments. The outflow Fe(III) concentrations reached steady-state within 6 hours to 2.5 days (1–5 pore volumes) after each change in Fe(III)-NTA input concentration and/or temperature setting (Fig. S1). In all FTR experiments, Fe(III)RRs increased with increasing Fe(III) input concentration and increasing temperature (Fig. 2). To assess whether our Fe(III) reduction rates only depend on the Fe(III) concentration and follow a pseudo first-order rather than a Michaelis–Menten rate model, we examined the linear relationship between Fe(III)RRs relative to steady-state Fe(III) concentration (Fig. S2). Linear regression coefficients provided clear evidence that a simple first-order reduction rate did not describe our FTR data. Thus, our FTR data was best modeled using Michaelis–Menten kinetics (Fig. 2; Table S2). Fe(II) was detected in the outflow after 6 hours (0.5 pore volumes) for all FTR experiments and reached steady-state within 0.5–1.5 days (1–2.5 pore volumes) for most Fe(III) input concentrations and temperature settings (Fig. S1). Iron(II) export rates (Fe(II)ERs) increased with increasing Fe(III) input concentrations and exhibited a strong positive correlation with Fe(III)RRs across sites, depths and temperature (Fig. 3).
Average steady-state potential Fe(III) reduction rates (Fe(III)RR) as a function of the average Fe(III) steady-state concentration in the FTR at three temperatures a 6 °C, b 12 °C and c 18 °C and two soil depths (30 cm and 70 cm) for depressional and slope wetland. The lines represent fitting of Michaelis–Menten kinetics. The error bars represent the standard deviation of measured steady-state Fe(III) concentrations (x-axis) and potential Fe(III) reduction rates (y-axis). When not visible, the error bars are within the size of the symbol
Correlation between steady-state potential Fe(III) reduction rates and Fe(II) export rates (nmol cm3 h−1) for the slope and depressional wetlands measured at 6, 12 and 18 °C. The dashed lines are the linear regressions (r2) representing the correlation between Fe(III) reduction rate and Fe(II) export rate for all wetland soils and depths. Error bars represent standard deviations for the calculated rates from steady-state Fe(III) and Fe(II) concentrations in the outflow
Kinetics of microbial Fe(III) reduction rates
Apparent maximum Fe(III) reduction rates (Vmax) ranged from 25 to 688 nmol cm−3 h−1 including all study sites and temperature settings (Fig. 4). Across wetland types, sites and depths, Vmax values at 18 °C were significantly higher than those at 6 and 12 °C (P < 0.05). The Vmax values were always higher at 30 cm than at 70 cm soil depth at the same wetland site (Fig. 4). For the depressional wetland, the response of Vmax to temperature varied among sites and depths. At 6 and 18 °C, the Vmax values at 30 cm depth were nearly similar for the center and transition sites, however, Vmax at 12 °C was 4 times higher at the center than transition site (Fig. 4). In addition, we observed statistically significant correlations between Vmax and soil porosity and dry bulk density (Table S3). Apparent Km values varied between wetland types, sites and soil depths ranging from 0.8 to 13.6 mM Fe(III) (Fig. 5). For all wetland soils, Km values at 18 °C were significantly higher than at 12 °C (P < 0.01) (Fig. 5). The kinetic data of Fe(III) reduction from duplicate FTR experiments and duplicate soil cores showed similar Vmax and Km for all three temperature settings for both the slope (P = 0.53) and depressional wetland (P = 0.83) (Fig. S3). Likewise, the temperature sensitivity of Vmax for these duplicate experiments were nearly identical (Fig. S3). The soil properties TN and Corg significantly affected the Km values at all wetland sites. (P < 0.05; Supplementary Table S3).
Temperature response of apparent maximum Fe(III) reduction rates (Vmax) and temperature dependence of Vmax as activation energy (Ea kJ mol−1) and temperature coefficient (Q10) for depressional and slope wetland soils. Apparent Vmax values are derived from FTR experiments at 6, 12 and 18 °C for two soil depths (30 cm, top panel and 70 cm, bottom panel). The grey area corresponds to the average apparent Vmax values of all sampling sites and depths at each temperature
Temperature response of apparent Fe(III) affinity constant (Km) derived from the FTR experiments at 6, 12 and 18 °C for two soil depths (30 cm, top panel and 70 cm, bottom panel) in depressional and slope wetland soils. The grey area corresponds to the averaged apparent Km values of all sampling sites and depths at each temperature
The temperature sensitivity of Vmax, expressed as temperature coefficient (Q10) and activation energy (Ea) did not differ significantly between wetland types (P = 0.29) and depths (P = 0.22; Fig. 4). The Q10 values varied between 1.5 and 4.2, except for the 70 cm slope wetland soil, which had a much higher value of 8.9 (Fig. 4). Across the depressional wetland, Ea and Q10 at 30 cm depth were nearly similar for all three sites (Fig. 4) with values ranging between 57 and 70 kJ mol−1 for Ea and 2.4 and 2.8 for Q10. The temperature coefficient Q10 was significantly influenced by the physico-chemical soil properties at both soil depths (Table 2). Q10 increased significantly (P < 0.05) with decreasing reactive Fe(III) phase at 30 cm soil depth. Q10 values were marginally related to both Corg/TN (P = 0.06) and TN (P = 0.08) at 30 cm soil depth. At 70 cm soil depth, Q10 increased as Corg/TN declined (P < 0.05) and the correlation between Corg and Q10 was marginal (P < 0.1; Table 2).
Discussion
Method validation for Fe(III) reduction kinetics
Both depressional and slope-type subalpine wetlands show a potential for microbial Fe(III) reduction, irrespective of location within the wetland or soil depth. As microbial degradation of complexed NTA does not take place in the absence of O2 (Wang et al. 2008; Lovley and Woodward 1996), NTA supply was unlikely to have promoted microbial growth of Fe(III)-reducing soil bacteria in our anaerobic FTR experiments. Also, as no Fe(II) was supplied in the input solution, its release from the FTRs corresponds to the production of Fe(II) as a result of microbial Fe(III) reduction. The strong correlation (0.56 > r2 = < 0.85) between Fe(II)ERs and Fe(III)RRs observed for all experiments indicates that Fe(III) reduction and Fe(II) production are nearly concomitant (Fig. 3). Further, the good agreement between the Fe(II)ERs and Fe(III)RRs suggests that the retention of Fe(III) on cation exchange sites within the soil and Fe(III) precipitation inside the reactor play a relatively minor role. All Fe(III) complexed with NTA is reduced and released as soluble Fe(II)-NTA without breaking the bond with NTA (Wang et al. 2008). The continuous discharge of weakly sorbing uncharged Fe(II)-NTA (Urrutia et al. 1999) inhibits the retention of Fe(II) by adsorption and precipitation inside the reactor. Thus, the Fe(II)ERs represent the maximum export of Fe(II) from the reactor. Under natural wetland conditions with lower discharge such as for the depressional wetland, we expect that a large fraction of dissolved Fe(II) adsorbs on soil oxide minerals (Urrutia et al. 1999), bacterial cells (Liu et al. 2002), and natural organic matter (Daugherty et al. 2017).
Effect of temperature on apparent Vmax and Km
Our results demonstrate that the apparent kinetic parameters for microbial Fe(III) reduction are temperature sensitive and an increase in temperature is paralleled by an increase in Vmax. Both apparent Vmax and Km are significantly different at 18 °C relative to 6 and 12 °C (P < 0.05), suggesting that microbial Fe(III) reduction is likely to change seasonally in these subalpine wetlands. The observed temperature sensitivity of the apparent kinetic parameters is linked either to physiological changes within the existing soil microbial community or conformation of enzymes with higher temperature (Wallenstein et al. 2010; Bradford 2013). Considering the low mean annual temperature (0.6 °C air temperature) at the study sites (Fig. 1), we assume that the soil microbial communities are dominated by psychrophilic and psychrotolerant microorganisms, which have an optimum growth temperature below 15 °C and do not grow above 20 °C (e.g., D’Amico et al. 2006). More specifically, optimum growth temperature for psychrophilic Fe(III)-reducing bacteria isolated from Arctic sediments was estimated to be 14 °C (Vandieken et al. 2006). Therefore, temperature above the optimum can affect the conformational enzyme structure (Bradford 2013) resulting in lower Fe(III) affinity without any significant change in the composition of the microbial soil community. In this study, a temperature increase from 6 to 12 °C presumably enhances the activity of functionally similar enzymes (higher Vmax) of psychrophilic and psychrotolerant microorganisms but without significant change in Fe(III) affinity. As a result, the positive correlation between Km, and the soil properties Corg and TN (Table S3) is potentially linked to the functional and physiological changes in Fe(III)-reducing soil communities with increasing temperature as organic substrates are required for the microbial Fe(III) reduction to take place.
Our study is the first to obtain apparent Km and Vmax for the reduction of organically-complexed Fe(III) (as Fe(III)-NTA) by complex soil microbial communities in intact soil cores that better approximate Fe dynamics in natural ecosystems than pure culture or slurry experiments. The Fe(III) in Fe(III)-NTA is highly available to soil bacteria (Bonneville et al. 2004). This explains why our Km values are one order of magnitude higher than Km values reported for most solid Fe(III) forms, such as reduction of amorphous Fe(III), hematite and goethite by Shewanella putrefaciens (Bonneville et al. 2004). However, about one-fifth of the Fe in soils and sediments is found in soluble Fe(III) complexed with organic matter that, like Fe(III)-NTA is highly accessible to soil bacteria (Gustafsson et al. 2007; Karlsson et al. 2008; Lalonde et al. 2012; Lovley and Woodward 1996). Consequently, our apparent Km and Vmax likely describe realistic in situ Fe reduction rates under optimal and higher-end boundary conditions.
Our Km values for Fe(III)-NTA reduction by wetland soil microbial communities were 3-4 orders of magnitude higher than reported for Shewanella species grown with Fe(III)-NTA (Liu et al. 2002; Table 3). Bacteria used in pure culture studies are typically selected for their high affinity for Fe(III) and generate Km values that may not accurately represent environmental conditions. In contrast, our approach aggregates the Km values of all organisms capable of utilizing Fe(III) as a terminal electron acceptor. The high abundance of culturable Fe(III)-reducing bacteria and high Vmax we measured are evidence that soils of both wetland types have high potential for Fe(III) reduction. The abundance of culturable Fe(III)-reducing bacteria in the study wetlands exceeded that of other wetland soils by up to two orders of magnitude (Roden and Wetzel 1996, 2002; Weiss et al. 2004). In addition, due to transport limitations that occur in soils, substrate accessibility is lower for particle-bound soil microbial communities compared to cell suspensions or slurry experiments. Consequently, substrate affinities in batch experiments with bacterial pure cultures are likely overestimated since mass transfer and Fe(III) accessibility are maximized.
The availability of reactive Fe(III) (Table 1) controls in situ Fe(III) reduction potential and is different for the two wetland types. In the case of the slope wetland, reactive soil Fe(III) measured for the two soil depths were greater than the determined Km values. This implies that microbial Fe(III) reduction is not limited by Fe availability under field conditions in the slope wetland soils. In contrast, at the depressional wetland reactive soil Fe(III) is lower than the determined Km values, indicating that Fe(III)-reducing bacteria operate below their maximum potential rate. Consequently, in situ Fe(III) reduction in the depressional wetland soils follows a first-order reaction with respect to ambient reactive soil Fe(III) indicating potential Fe(III) limitation to soil bacteria.
Factors controlling variation in Q10 and Ea values
The large variations in Q10 and Ea within subalpine wetland soils compared to other ecosystems (Table 3) suggest that a single value for temperature sensitivity is insufficient to quantify the seasonal temperature change for predictive Fe(III) reduction models. Our apparent Q10’s for Fe(III) reduction (average Q10 = 3.5) are comparable to those measured for other microbially-mediated redox processes in other cold and temperate ecosystems. For example, Q10 of 3.9 were reported for sulfate reduction (2–12 °C) in cold marine sediments (Isaksen and Jorgensen 1996) and Q10 values between 3.4 and 5.6 were reported for soil respiration in temperate forest soils (Davidson et al. 1998). The Q10’s for Fe(III) reduction in the subalpine wetland soils are slightly higher than those reported for microbial Fe(III) reduction in temperate (Table 3; Meier et al. 2005) and subtropical lake sediments (Table 3; Chen et al. 2014). Further, apparent Ea values for Fe(III) reduction obtained here for subalpine wetland soils show larger variation than observed for temperate freshwater wetland (44–52 kJ mol−1; Roden and Wetzel 1996) and lake sediments (42–46 kJ mol−1; Meier et al. 2005). This indicates that Fe cycling in subalpine ecosystems is more temperature sensitive than in warmer climates, a finding consistent with the observation that the temperature sensitivity of microbially-mediated soil reactions is greater in colder compared to warmer climates (German et al. 2012).
The variability in Q10 (1.5–8.9) and Ea values (26–148 kJ mol−1) among the studied wetland soils reflects the variations in Corg/TN and reactive soil Fe(III). We found that reactive soil Fe(III) is the primary control on Q10 in soils from 30 cm depth at both wetlands. Q10 is almost twice as high in soils with the lowest compared to the highest reactive Fe(III) content (22.3 µmol g−1 vs. 376 µmol g−1). This suggests that microbial Fe(III) reduction is more temperature sensitive when reactive soil Fe(III) is low. Its correlation with Q10 suggests that microbes may first utilize available, reactive Fe(III) during short-term warming fluctuations, such as experienced during seasonal change. On the other hand, the higher Q10 values observed in the 70 cm depth soils are significantly influenced by Corg/TN content.
Implication for microbial Fe(III) reduction in wetlands
Our study provides a first approximation of how seasonal temperature change can affect microbial Fe(III) reduction in subalpine wetland soils. Maximum Fe(III) reduction rates (Vmax) follow an expected seasonal pattern in both subalpine wetlands. The significantly higher Vmax measured at 18 °C demonstrate that Fe cycling is likely to accelerate during warmer summer days in these subalpine wetlands.
Temperature sensitivities of microbially-mediated processes vary (Wallenstein et al. 2010) when the composition and enzymatic reaction rates of microbial soil assemblages change seasonally. The observed changes in Vmax and Km with temperature for soils of both subalpine wetlands emphasize the importance of deriving temperature-specific Fe(III) reduction kinetics to predict dissimilatory Fe(III) reduction for seasonal temperature variations or in wetlands located in different temperature regimes. The apparent Km and Vmax values for the 6 °C FTR experiments exhibit most likely Fe(III) reduction kinetics for temperature conditions during late spring and early autumn (Fig. 1; Elder 2006) with predominantly psychrophilic Fe(III)-reducing bacteria. The apparent Km and Vmax values at 12 °C FTR correspond to the highest mean temperature of 12.2 °C in July (Fig. 1; Elder 2006) and seems to represent the optimum temperature for psychrophilic Fe(III)-reducing bacteria. Accordingly, our results are the first step toward linking in situ Fe(III) reduction kinetics with potential seasonal temperature variation.
Depth-related variation in soil properties on Q10 and Ea indicates that spatial heterogeneity in temperature sensitivity for microbial Fe(III) reduction should be integrated into projections of the impacts of seasonal temperature change. Our findings on temperature sensitivity (Q10 and Ea) for microbial Fe(III) reduction could be extrapolated to other cold or temperate, water-saturated ecosystems with similar seasonal pattern. Fe(III) reduction reactions could be particularly important in environments where Fe(III) reduction inhibits CH4 production (Kruger et al. 2001; Kogel-Knabner et al. 2010; Herndon et al. 2015) though such extrapolation requires caution. For example, our laboratory-based approach reflects short-term temperature responses that do not account for longer-term changes related to acclimation of microbial soil communities to changing environmental conditions. Future studies may address if changes in physiology and taxa of soil microbial community and acclimation affects the Fe(III) reduction kinetics and Q10. Our findings are a step toward improved understanding of the response of microbial Fe(III) reduction kinetics to increasing temperature and how temperature sensitivity differs among wetland types and with varying soil properties.
A thorough knowledge of the causes for the variability in Fe(III) reduction kinetics and temperature sensitivity in wetland ecosystems can be used to evaluate the release of dissolved organic carbon and nutrients (e.g., phosphorous) due to reductive Fe(III) dissolution. Thus, our results on Fe(III) reduction kinetics have direct implications for tracing the cycling of dissolved organically-complexed Fe(III) and dissolved Corg. As approximately one-fifth of Fe in the environment is complexed with organic matter (Gustafsson et al. 2007; Karlsson et al. 2008; Lalonde et al. 2012), up to 14 times higher Vmax at 18 °C than at 6 °C accelerates the reductive dissolution of Fe(III) and the release of organically-complexed Fe(II) from soils to the solution. Such change in the Fe redox state influences the stabilization of soil C pools due to interactions between Fe-bearing minerals and soil organic matter (Borch et al. 2010; Wagai and Mayer 2007). Although Fe(III) reduction kinetics in this study reflect only the temperature response of microbial reduction of dissolved organically-complexed Fe(III), our data can be used as high-end boundary kinetic values for Fe(III) reduction to optimize Fe cycling models.
Conclusion
Our experiments demonstrate that even short-term temperature increase induce changes in dissimilatory Fe(III) reduction kinetics of organically complexed Fe(III). Indeed, temperature increase governs microbial Fe(III) reduction directly by changes in Fe(III) reduction kinetics (Vmax and Km) at both subalpine wetland types. Soil Corg/TN and reactive Fe content are significant factors regulating the variation in Q10 and Ea within the wetland soils. In conclusion, our study provides a much-needed approach for determining how seasonal temperature variations affect Fe(III) cycling under environmental and soil-physical conditions relevant for various wetland types.
References
Amstaetter K, Borch T, Kappler A (2012) Influence of humic acid imposed changes of ferrihydrite aggregation on microbial Fe(III) reduction. Geochim Cosmochim Acta 85:326–341
Atkin OK, Edwards JE, Loveys BR (2000) Response of root respiration to changes in temperature and its relevance to global warming. New Physil 147:141–154
Bååth E (2018) Temperature sensitivity of soil microbial activity modeled by the square root equation as a unifying model to differentiate between direct temperature effects and microbial community adaptation. Glob Chang Biol 24:2850–2861
Baldwin DS, Rees GN, Mitchell AM, Watson G, Williams J (2006) The short-term effects of salinization on anaerobic nutrient cycling and microbial community structure in sediment from a freshwater wetland. Wetlands 26:455–464
Belotte D, Curien JB, Maclean RC, Bell G (2003) An experimental test of local adaptation in soil bacteria. Evolution 57:27–36
Blagodatskaya E, Blagodatsky S, Khomyakow N, Myachina O, Kuzyakova Y (2015) Temperature sensitivity and enzymatic mechanisms of soil organic matter decomposition along an altitudinal gradient on Mount Kilimanjaro. Sci Rep 6:22240
Blake GR, Hartge KH (1986) Bulk density. In: Methods of soil analysis. Part 1-Physical and mineralogical methods. American Society of Agronomy-Soil Science Society of America, Madison, pp 363–375
Bonneville S, Van Cappellen P, Behrends T (2004) Microbial reduction of iron(III) oxyhydroxides: effects of mineral solubility and availability. Chem Geol 212:255–268
Borch T, Kretzschmar R, Kappler A, Van Cappellen P, Ginder-Vogel M, Voegelin A, Campbell K (2010) Biogeochemical redox processes and their impact on contaminant dynamics. Environ Sci Technol 44:15–23
Bradford MA (2013) Thermal adaptation of decomposer communities in warming soils. Front Microbiol 4:1–16
Brinson MM (1993) A hydrogeomorphic classification for wetlands. Wetlands Research Program, U.S. Army Corps of Engineers Waterways Experiment Station, Vicksburg, MS, USA. Technical Report WRP-DE-4
Buford Price P, Sowers T (2004) Temperature depedence of metabolic rates for microbial growth, maintenance and survival. Proc Natl Acad Sci 101:4631–4636
Carsey K, Kittel G, Decker K, Cooper DJ, Culver DR (2003) Field guide to the wetland and riparian plant associations of Colorado. Colorado National Heritage Program. Colorado State University, Fort Collins
Chen M, Ye T-R, Krumholz LR, Jian H-L (2014) Temperature and cyanobacterial bloom biomass influence phosphorous cycling in eutrophic lake sediments. PLoS 9:1–10
D’Amico S, Collins T, Marx JC, Feller G, Gerday C (2006) Psychrophilic microorganisms: challenges for life. EMBO Rep 7:385–389
Daugherty EE, Gilbert B, Nico PS, Borch T (2017) Complexation and redox buffering of iron(II) by dissolved organic matter. Environ Sci Technol 51:11096–11104
Davidson EA, Belk E, Boone RD (1998) Soil water content and temperature as independent or confound factors controlling soil respiration in a temperate mixed hardwood forest. Glob Change Biol 4:217–227
Davidson EA, Janssens IA, Luo Y (2006) On the variability of respiration in terrestrial ecosystems: moving beyond Q10. Glob Change Biol 12:154–164
Dong Y, Somero GN (2009) Temperature adaptation of cytosolic malate dehydrogenases of limpets (genus Lottia): differences in stability and function due to minor changes in sequence correlate with biogeographic and vertical distributions. J Exp Biol 212:169–177
Elder K (2006) Fraser experimental forest headquarters station hourly temperature data: 1976–2003. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fort Collins. https://doi.org/10.2737/RDS-2006-0002
German DP, Marcelo KRB, Stone MM, Allison SD (2012) The Michaelis-Menten kinetics of soil extracellular enzymes in response to temperature: a cross-latitudinal study. Glob Change Biol 18:1468–1479
Gu C, Laverman AM, Pallud C (2012) Environmental controls on nitrogen and sulfur cycles in surficial aquatic sediments. Front Microbiol 3:45
Gustafsson JP, Persson I, Kleja DB, van Schaik JWJ (2007) Binding of iron(III) to organic soils: EXAFS spectroscopy and chemical equilibrium modeling. Environ Sci Technol 41(4):1232–1237
Hall SJ, Berhe AA, Thompson A (2018) Order from disorder: do soil organic matter composition and turnover co-vary with iron phase crystallinity? Biogeochemistry 140:93–110
Herndon EM, Yang ZM, Bargar J, Janot N, Regier T, Graham D, Wullschleger S, Gu BH, Liang LY (2015) Geochemical drivers of organic matter decomposition in arctic tundra soils. Biogeochemistry 126:397–414
Hyacinthe C, Bonneville S, Van Cappellen P (2006) Reactive iron(III) in sediments: chemical versus microbial extractions. Geochim Cosmochim Acta 70:4166–4180
Isaksen MF, Jorgensen BB (1996) Adaptation of psychrophilic and psychrotrophic sulfate-reducing bacteria to permanently cold marine environments. Appl Environ Microbiol 62:408–414
Johnston SG, Burton ED, Aaso T, Tuckerman G (2014) Sulfur, iron and carbon cycling following hydrological restoration of acidic freshwater wetlands. Chem Geol 371:9–26
Karlsson T, Persson P, Skyllberg U, Mörth C-M, Giesler R (2008) Characterization of iron(III) in organic soils using extended X-ray absorption fine structure spectroscopy. Environ Sci Technol 42:5449–5454
Kogel-Knabner I, Amelung W, Cao ZH, Fiedler S, Frenzel P, Jahn R, Kalbitz K, Kolbl A, Schloter M (2010) Biogeochemistry of paddy soils. Geoderma 157:1–14
Kruger M, Frenzel P, Conrad R (2001) Microbial processes influencing methane emission from rice fields. Glob Change Biol 7:49–63
Lalonde K, Mucci A, Ouellet A, Gelinas Y (2012) Preservation of organic matter in sediments promoted by iron. Nature 483:198–200
LaPerriere Nelson M, Rhoades CC, Dwire KA (2011) Influence of bedrock geology on water chemistry of slope wetlands and headwater streams in the Southern Rocky Mountains. Wetlands 31:251–261
Laverman AM, Van Cappellen P, Van Rotterdam-Los D, Pallud C, Abell J (2006) Potential rates and pathways of microbial nitrate reduction in coastal sediments. FEMS Microbiol Ecol 58:179–192
Laverman AM, Pallud C, Abell J, Van Cappellen P (2012) Comparative survey of nitrate and sulfate reduction in aquatic sediments. Geochim Cosmochim Acta 77:474–488
Liu C, Gorby YA, Zachara JM, Fredrickson JK, Brown CF (2002) Reduction kinetics of Fe(III), Co(III), U(VI), Cr(VI), and Tc(VII) in cultures of dissimilatory metal-reducing bacteria. Biotechnol Bioeng 80:637–649
Lovley DR, Phillips EJP (1991) Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev 55:259–287
Lovley DR, Woodward JC (1996) Mechanisms for chelator stimulation of microbial Fe(III)-oxide reduction. Chem Geol 132:19–24
Lovley DR, Holmes DE, Nevin KP (2004) Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microb Physiol 49:219–286
Meier J, Costa R, Smalla K, Boehrer B, Wendt-Potthoff K (2005) Temperature dependence of Fe(III) and sulfate reduction rates and its effect on growth and composition of bacterial enrichments from an acidic pit lake neutralization experiment. Geobiology 3:261–274
Morton SC, Zhang Y, Edwards MA (2005) Implications of nutrient release from iron metal from microbial regrowth in water distribution system. Water Res 39:2883–2892
Nelson DW, Sommers LE (1986) Total carbon, organic carbon and organic matter. In: Methods of soil analysis, part 3, chemical methods. Am Soc Argon 961–1010
Pallud C, Van Cappellen P (2006) Kinetics of microbial sulfate reduction in estuarine sediments. Geochim Cosmochim Acta 70:1148–1162
Pallud C, Meile C, Laverman AM, Abell J, Van Cappellen P (2007) The use of flow-through sediment reactors in biogeochemical kinetics: methodology and examples of applications. Mar Chem 106:256–271
Pan W, Kan J, Inamdar S, Chen C, Sparks D (2016) Dissimilatory microbial iron reduction release DOC (dissolved organic carbon) from carbon-ferrihydrite association. Soil Biol Biochem 103:232–240
Richards CM, Pallud C (2016) Kinetics of sulfate reduction and sulfide precipitation rates in sediments of a bar-built estuary (Pescadero, California). Water Res 94:86–102
Riedel T, Zak D, Biester H, Dittmar T (2013) Iron traps terrestrially derived dissolved organic matter at redox interfaces. PNAS 110:10101–10105
Roden EE (2003) Fe(III) oxide reactivity toward biological versus chemical reduction. Environ Sci Technol 37:1319–1324
Roden EE, Wetzel RG (1996) Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol Oceanogr 41:1733–1748
Roden EE, Wetzel RG (2002) Kinetics of microbial Fe(III) oxide reduction in freshwater wetland sediments. Limnol Oceanogr 41:1733–1748
Roychoudhury AN, Viollier E, Van Cappellen P (1998) A plug flow-through reactor for studying biogeochemical reactions in undisturbed aquatic sediments. Appl Geochem 13:269–280
Sanford RA, Wu Q, Sung Y, Thomas SH, Amos BK, Prince EK, Löffler FE (2007) Hexavalent uranium supports growth of Anaeromyxobacter dehalogenans and Geobacter spp. with lower than predicted biomass yields. Environ Microbiol 9(11):2885–2893
Schilling K, VillaRomero JF, Pallud C (2018) Selenate reduction rates and kinetics across depth in littoral sediment of the Salton Sea, California. Biogeochemistry 140:285–298
Schipper LA, Hobbs JK, Rutledge S, Arcus VL (2014) Thermodynamic theory explains the temperature optima of soil microbial processes and high Q10 values at low temperature. Glob Chang Biol 20:3578–3586
Shimizu M, Zhou J, Schröder C, Obst M, Kappler A, Borch T (2013) Dissimilatory reduction and transformation of ferrihydrite-humic acid coprecipitates. Environ Sci Technol 47:13375–13384
Siddiqui KS, Cavicchioli R (2006) Cold-adapted enzymes. Annu Rev Biochem 75:403–433
Slomp CP, Malschaert H, Lohse L, Van Raaphorst W (1997) Iron and manganese cycling in different sedimentary environment on the North Sea continental margin. Cont Shelf Res 17:1083–1117
Stam M, Mason P, Pallud C, Van Cappellen P (2010) Sulfate reducing activity and sulfur isotope fractionation by natural communities in sediments of a hypersaline soda lake (Mono Lake, CA). Chem Geol 278:23–30
Stam MC, Mason PRD, Laverman AM, Pallud C, Van Cappellen P (2011) 34S/32S fractionation by sulfate-reducing microbial communities in estuarine sediments. Geochim Cosmochim Acta 75:3903–3914
Stookey LL (1970) Ferrozine—a new spectrophotometric reagent for iron. Anal Chem 42:779–781
Tarpgaard IH, Røy H, Jørgensen BB (2011) Concurrent low- and high-affinity sulfate reduction kinetics in marine sediment. Geochim Cosmochim Acta 75:2997–3010
Trott T, Henwood RW, Langford CH (1972) Sunlight photochemistry of ferric nitrilotriacetate complexes. Environ Sci Technol 6:366–368
Urrutia MM, Roden EE, Zachara JM (1999) Influence of aqueous and solid-phase Fe(II) complexants on microbial reduction of crystalline iron(III) oxides. Environ Sci Technol 33:4022–4028
Vandieken V, Finke N, Jorgensen BB (2006) Pathways of carbon mineralization for an Arctic fjord sediment (Svalbard) and isolation of psychrophilic and psychrotolerant, iron reducing bacteria. Mar Ecol Prog Ser 322:29–41
Wagai R, Mayer LM (2007) Sorptive stabilization of organic matter in soils by hydrous iron oxides. Geochim Cosmochim Acta 71:25–35
Wallenstein M, Allison SD, Ernakovich J, Steinweg JM, Sinsabaugh R (2010) Controls on the temperature sensitivity of soil enzymes: a key driver of in situ enzyme activity rates. In: Shukla G, Varma A (eds) Soil Enzymology. Springer, Berlin-Heidelberg, pp. 245–258
Wang Z, Lui C, Wang X, Marshall MJ, Zachara JM, Rosso KM, Dupuis M, Fredrickson JK (2008) Kinetics of reduction of Fe(III) complexes by outer membrane cytochromes MtrC and OmcA of Shewanella oneidensis MR-1. Appl Environ Microbiol 74:6746–6755
Weiss JV, Emerson D, Megonigal JP (2004) Geochemical control of microbial Fe(III) reduction potential in wetlands: comparison of the rhizosphere to non-rhizosphere soil. FEMS Microbiol Ecol 48:89–100
Wilson GV, Rhoton FE, Selim HM (2004) Modeling the impact of ferrihydrite on adsorption-desorption of soil phosphorus. Soil Sci 169:271–281
Woomer PL (1994) Most probable number counts. In: Methods of Soil Analysis. Part 2-Microbiological and Biochemical Properties. Soil Science Society of America Inc, USA, pp 59–79
Yang ZM, Wullschleger SD, Liang LY, Graham DE, Gu BH (2016) Effects of warming on the degradation and production of low-molecular-weight labile organic carbon in an Arctic tundra soil. Soil Biol Biochem 95:202–211
Zachara JM, Fredrickson JK, Smith SC, Gassman PL (2001) Solubilization of Fe(III) oxide-bound trace metals by a dissimilatory Fe(III) reducing bacterium. Geochim Cosmochim Acta 65:75–93
Zhao Q, Adhikari D, Huang R, Patel A, Wang X, Tang X, Obrist D, Roden EE, Yang Y (2016) Coupled dynamics of iron and iron-bound organic carbon in forest soils during anaerobic reduction. Chem Geol 464:118–126
Acknowledgement
We thank Chandra Richards for her FTR pilot study, Clifford Wang for analyzing Fe concentrations of the FTR experiment and Ellen Daugherty for her help with the soil core sampling. Funding for this project was provided by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2013-67019-21359 and, in part, by a National Science Foundation (NSF) Award (EAR 1451494) to T.B.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: R. Kelman Wieder.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Schilling, K., Borch, T., Rhoades, C.C. et al. Temperature sensitivity of microbial Fe(III) reduction kinetics in subalpine wetland soils. Biogeochemistry 142, 19–35 (2019). https://doi.org/10.1007/s10533-018-0520-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-018-0520-4