Abstract
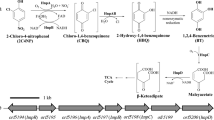
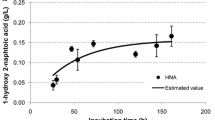
Sphingomonas sp. strain TTNP3 has been previously described as a bacterium that is capable of degrading the technical mixture of nonylphenol (NP) isomers and also the 4(3′,5′-dimethyl-3′-heptyl)-phenol single isomer of NP. Until recently, 3,5-dimethyl-3-heptanol was the only reported metabolite of 4(3′,5′-dimethyl-3′-heptyl)-phenol. A short time ago, the detection of an intracellular metabolite resulting from the oxidation of 4(3′,5′-dimethyl-3′-heptyl)-phenol which was identified as 2(3,5-dimethyl-3-heptyl)-benzenediol has been reported. A decisive element for this identification was the occurrence of some slight differences with the two most probable metabolites i.e. 4(3′,5′-dimethyl-3′-heptyl)-resorcinol and 4(3′,5′-dimethyl-3′-heptyl)-catechol. These facts led us to hypothesise some NIH shift mechanisms explaining the formation of 2(3′,5′-dimethyl-3′-heptyl)-benzenediol. In the present work, we describe the steps that led to the detection of these metabolites in the intracellular fraction of Sphingomonas sp. strain TTNP3. The formation of analogous intracellular metabolites resulting from the degradation of the technical mixture of NP is reported. To further elucidate these degradation products, studies were carried out with cells grown with 4(3′,5′-dimethyl-3′-heptyl)-phenol as sole carbon source. The description of the syntheses of reference compounds, i.e. 4(3′,5′-dimethyl-3′-heptyl)-resorcinol and 4(3′,5′-dimethyl-3′-heptyl)-catechol and their comparative analyses with the intermediates of the degradation of 4(3′,5′-dimethyl-3′-heptyl)-phenol are presented.
Similar content being viewed by others
Abbreviations
- CID:
-
collision-induced dissociation
- NP:
-
nonylphenol
- p353NC:
-
4(3′,5′-dimethyl-3′-heptyl)-catechol
- p353NP:
-
4(3′,5′-dimethyl-3′-heptyl)-phenol (nonylphenol)
- p353NR:
-
4(3′,5′-dimethyl-3′-heptyl)-resorcinol
- tNP:
-
technical nonylphenol
References
Bokern M, Nimtz M, Harms HH (1996) Metabolites of 4-n-nonylphenol in wheat cell suspension cultures. J␣Agric Food Chem 44:1123–1127
Corti A, Frassinetti S, Vallini G, D’Antone S, Fichi C,␣Solaro R (1995) Biotransformation of nonionic surfactants. I. Biotransformation of 4-(1-nonyl)phenol by Candida maltosa isolate. Environ Pollut 90:83–87
Corvini PFX, Vinken R, Hommes G, Schmidt B, Dohmann M (2004a) Degradation of the radioactive and non-labelled branched 3′,5′-dimethyl 3′-heptyl-phenol nonylphenol isomer by Sphingomonas TTNP3. Biodegradation 15:9–18
Corvini PFX, Vinken R, Hommes G, Mundt M, Meesters R, Schröder HF, Hollender J, Schmidt B (2004b) Microbial degradation of a single branched isomer of nonylphenol by Sphingomonas TTNP3. Water Sci Technol 50:195–202
Corvini PFX, Meesters RJW, Schäffer A, Schröder HFr, Vinken R, Hollender J (2004c) Degradation of a nonylphenol single isomer by Sphingomonas sp. strain TTNP3 leads to a hydroxylation-induced migration product. Appl Environ Microbiol 70:6897–6900
Dachs J, Van Ry DA, Eisenreich SJ (1999) Occurrence of estrogenic nonylphenols in the urban and coastal atmosphere of the lower Hudson River estuary. Environ Sci Technol 33:2676–2679
Di Corcia A, Costantino A, Crescenzi C, Marinoni E, Samperi R (1998) Characterization of recalcitrant intermediates of the branched alkyl side chain of nonylphenol ethoxylate surfactants. Environ Sci Technol 32:2401–2409
Ekelund R, Granmo Å, Magnusson K, Berggren M (1993) Biodegradation of 4-nonylphenol in seawater and sediment. Environ Pollut 79:59–61
Espadaler I, Caixach J, Om J, Ventura F, Cortina M, Pauné F, Rivera J (1997) Identification of organic pollutants in Ter river and its system of reservoirs supplying water to Barcelona (Catalonia, Spain): a study by GC/MS and FAB/MS. Water Res 31:1996–2004
Fereira-Leach AMR, Hill EM (2001) Bioconcentration and distribution of 4-tert-octylphenol residues in tissues of the rainbow trout (Oncorhynchus mykiss). Mar Environ Res 51:75–89
Fujii K, Urano N, Ushio H, Satomi M, Iida H, Ushio-Sata N, Kimura S (2000) Profile of a nonylphenol-degrading microflora and its potential for bioremedial applications. J Biochem 128:909–916
Fujii K, Urano N, Ushio H, Satomi M, Kimura S (2001) Sphingomonas cloacae sp. nov., a nonylphenol-degrading bacterium isolated from wastewater of a sewage-treatment plant in Tokyo. Int J Syst Evol Microbiol 51:603–610
Giger W, Ahel M, Koch M, Laubscher HU, Schaffner C, Schneider J (1987) Behaviour of alkylphenolpolyethoxylate surfactants and of nitrilotriacetate in sewage treatment. Water Sci Technol 19:449–460
Guroff G, Daly JW, Jerina DM, Renson J, Witkop B, Udenfriend S (1967) Hydroxylation-induced migration: the NIH shift. Science 157:1524–1530
Hesselsøe M, Jensen D, Skals K, Olesen T, Moldrup P, Roslev P, Krog Mortensen G, Henriksen K (2001) Degradation of 4-nonylphenol in homogeneous and nonhomogeneous mixtures of soil and sewage sludge. Environ Sci Technol 35:3695–3700
Imai Y, Matsunaga I, Kusunose E, Ichihara K (2000) Unique heme environment at the putative distal region of hydrogen peroxide-dependant fatty acid α-hydroxylase from Sphingomonas paucimobilis (peroxygenase P450SPα). J Biochem 128:189–194
Koerts J, Soffers AEMF, Vervoort J, De Jager A, Rietjens IMCM (1998) Occurrence of the NIH shift upon the cytochrome P450-catalyzed in vivo and in vitro aromatic ring hydroxylation of fluorobenzenes. Chem Res Toxicol 11:503–512
Lalah JO, Schramm KW, Severin GF, Lenoir D, Henkelmann B, Behechti A, Guenther K, Kettrup A (2003) In vivo metabolism and organ distribution of a branched 14C-nonylphenol isomer in pond snails, Lymnea stagnalis L. Aquat Toxicol 62:305–319
Liber K, Knuth ML, Stay FS (1999) An integrated evaluation of the persistence and effects of 4-nonylphenol in an experimental littoral ecosystem. Environ Toxicol Chem 18:357–362
Lobos JH, Leib TK, Su TM (1992) Biodegradation of bisphenol A and other bisphenols by a gram-negative bacterium. Appl Environ Microbiol 58:1823–1831
Soto AM, Justica H, Wray JW, Sonnenschein C (1991) Para-nonylphenol: an estrogenic xenobiotic released from polystyrene. Environ Health Perspect 92:167–173
Spivack J, Leib TK, Lobos JH (1994) Novel pathway for bacterial metabolism of bisphenol A. J Biol Chem 269:7323–7329
Sundaram KMS, Szeto S (1981) The dissipation of nonylphenol in stream and pond water under simulated field conditions. J Environ Sci Health B16:767–776
Tanghe T, Devriese G, Verstraete W (1998) Nonylphenol degradation in lab scale activated sludge units is temperature dependent. Water Res 32:2889–2896
Tanghe T, Dhooge W, Verstraete W (1999) Isolation of a bacterial strain able to degrade branched nonylphenol. Appl Environ Microbiol 65:746–751
Tanghe T, Dhooge W, Verstraete W (2000) Formation of the metabolic intermediate 2,4,4-trimethyl-3-pentanol during incubation of a Sphingomonas sp. strain with the xeno-estrogenic octylphenol. Biodegradation 11:11–19
Topp E, Starratt A (2000) Rapid mineralization of the endocrine-disrupting chemical 4-nonylphenol in soil. Environ Toxicol Chem 19:313–318
Ushiba Y, Takahara Y, Ohta H (2003) Sphingobium amiense sp. nov., a novel nonylphenol-degrading bacterium isolated from a river sediment. Int J Syst Evol Microbiol 53:2045–2048
Vallini G, Frassinetti S, Scorzetti G (1997) Candida aquaetextoris sp. nov., a new species of yeast occurring in sludge from a textile industry wastewater treatment plant in Tuscany, Italy. Int J Syst Bacteriol 47:336–340
Van Ginkel CG 1996. Complete degradation of xenobiotic surfactants by consortia of aerobic microorganisms. Biodegradation 7:151–164
Vinken R, Schmidt B, Schäffer A (2002) Synthesis of tertiary 14C-labelled nonylphenol isomers. J Label Compd Radiopharm 45:1253–1263
Wheeler TF, Heim JR, LaTorre MR, B Janes (1997) Mass spectral characterization of p-nonylphenol isomers using high-resolution capillary GC-MS. J Chromatogr Sci 35:19–30
Acknowledgements
The authors wish to thank Dr J. Runsink and Mrs A. Müller for measurement of the NMR spectra at the Institute for Organic Chemistry and Maike Meindorf for her technical support. The work of LabMET was made possible by a grant from the FWO (Flemish Fund Scientific Research) n° G.0102.00N.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corvini, P.F.X., Meesters, R., Mundt, M. et al. Contribution to the Detection and Identification of Oxidation Metabolites of Nonylphenol in Sphingomonas sp. strain TTNP3. Biodegradation 18, 233–245 (2007). https://doi.org/10.1007/s10532-006-9058-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-006-9058-6