Abstract
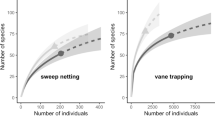
Across Europe conservation actions have been implemented to mitigate the decline of pollinators in agricultural landscapes. However, recent concerns have appeared about their efficiency to promote pollinator diversity. To increase the efficiency of these interventions, one must acquire a better knowledge of the target species diversity patterns and its sources of variations at different spatial and temporal scales. This study sets out to identify the main sources of variation in wild bee assemblages at a regional scale (450 km2) in mass-flowering crops and semi-natural habitats. During three consecutive sampling years, we monitored bee diversity and its temporal and spatial turnovers. We show that an intensive agricultural landscape in western France can hold nearly 200 wild bee species at a regional scale, i.e. 20 % of the whole bee fauna known in mainland France. Wild bee diversity was 3–4 times lower in oleaginous crops than in semi-natural habitats, with a substantial number of these being social and gregarious species. Spatial and seasonal species turnover in semi-natural habitats explained 28.6 and 34.3 %, respectively, of regional species richness. Given the importance of the spatial component of the bee diversity turnover, we suggest wild bee conservation efforts should be carried out at relevant spatial scales. The spatial turnover was estimated to be steeper within 50 km2 scales. This provides an order of magnitude for the spatial extent of relevant conservation units within which one may concentrate conservation efforts in order to optimise the number of species promoted per surface area.





Similar content being viewed by others
References
Amiet F, Müller A, Neumeyer R (1999) Apidae 2: Colletes, Dufourea, Hylaeus, Nomia, Nomioides, Rhophitoides, Rophites, Sphecodes, Systropha. Centre Suisse de Cartographie de la Faune, Neuchâtel
Amiet F, Herrmann M, Müller A, Neumeyer R (2001) Apidae 3: Halictus, Lasioglossum. Centre Suisse de Cartographie de la Faune, Neuchâtel
Amiet F, Herrmann M, Müller A, Neumeyer R (2004) Apidae 4. Anthidium, Chelostoma, Coelioxys, Dioxys, Heriades, Lithurgus, Megachile, Osmia, Stelis. Centre Suisse de Cartographie de la Faune, Neuchâtel
Amiet F, Herrmann M, Müller A, Neumeyer R (2007) Apidae 5. Ammobates, Ammobatoides, Anthophora, Biastes, Ceratina, Dasypoda, Epeoloides, Epeolus, Eucera, Macropis, Melecta, Melitta, Nomada, Pasites, Tetralonia, Thyreus, Xylocopa. Centre Suisse de Cartographie de la Faune, Neuchâtel
Amiet F, Herrmann M, Müller A, Neumeyer R (2010) Apidae 6: Andrena, Melitturga, Panurginus, Panurgus. Centre Suisse de Cartographie de la Faune, Neuchâtel
Biesmeijer JC, Roberts SPM, Reemer M et al (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313:351–354. doi:10.1126/science.1127863
Cameron SA, Lozier JD, Strange JP et al (2011) Patterns of widespread decline in North American bumble bees. Proc Natl Acad Sci 108:662–667. doi:10.1073/pnas.1014743108
Carvalheiro LG, Seymour CL, Veldtman R, Nicolson SW (2010) Pollination services decline with distance from natural habitat even in biodiversity-rich areas. J Appl Ecol 47:810–820. doi:10.1111/j.1365-2664.2010.01829.x
Chase JM (2011) Ecological niche theory. the theory of ecology. University of Chicago Press, Chicago, p 416
Chave J (2004) Neutral theory and community ecology. Ecol Lett 7:241–253. doi:10.1111/j.1461-0248.2003.00566.x
Colwell RK (2013) Estimates: statistical estimation of species richness and shared species from samples. Divers Distrib 14:1–10
Crist TO, Veech JA (2006) Additive partitioning of rarefaction curves and species–area relationships: unifying α-, β- and γ-diversity with sample size and habitat area. Ecol Lett 9:923–932. doi:10.1111/j.1461-0248.2006.00941.x
Crist TO, Veech JA, Gering JC, Summerville KS (2003) Partitioning species diversity across landscapes and regions: A hierarchical analysis of α, β, and γ diversity. Am Nat 162:734–743
Dengler J (2009) Which function describes the species–area relationship best? A review and empirical evaluation. J Biogeogr 36:728–744. doi:10.1111/j.1365-2699.2008.02038.x
R Development Core Team (2010) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Dicks LV, Showler DA, Sutherland WJ (2010) Bee conservation: Evidence for the effects of interventions, exeter. Pegasus Publishing, Cambridge
Duelli P, Obrist MK (2003) Regional biodiversity in an agricultural landscape: the contribution of seminatural habitat islands. Basic Appl Ecol 4:129–138. doi:10.1078/1439-1791-00140
Gering JC, Crist TO, Veech JA (2003) Additive partitioning of species diversity across multiple spatial scales: implications for regional conservation of biodiversity. Conserv Biol 17:488–499. doi:10.1046/j.1523-1739.2003.01465.x
Goulson D, Lepais O, O’Connor S et al (2010) Effects of land use at a landscape scale on bumblebee nest density and survival. J Appl Ecol 47:1207–1215. doi:10.1111/j.1365-2664.2010.01872.x
Greenleaf SS, Williams NM, Winfree R, Kremen C (2007) Bee foraging ranges and their relationship to body size. Oecologia 153:589–596. doi:10.1007/s00442-007-0752-9
Hoehn P, Steffan-Dewenter I, Tscharntke T (2010) Relative contribution of agroforestry, rainforest and openland to local and regional bee diversity. Biodivers Conserv 19:2189–2200. doi:10.1007/s10531-010-9831-z
Holzschuh A, Dormann CF, Tscharntke T, Steffan-Dewenter I (2013) Mass-flowering crops enhance wild bee abundance. Oecologia 172:477–484. doi:10.1007/s00442-012-2515-5
Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography. Princeton University Press, Princeton
Jauker F, Peter F, Wolters V, Diekötter T (2012) Early reproductive benefits of mass-flowering crops to the solitary bee Osmia rufa outbalance post-flowering disadvantages. Basic Appl Ecol 13:268–276. doi:10.1016/j.baae.2012.03.010
Jauker B, Krauss J, Jauker F, Steffan-Dewenter I (2013) Linking life history traits to pollinator loss in fragmented calcareous grasslands. Landscape Ecol 28:107–120. doi:10.1007/s10980-012-9820-6
Kirk WDJ, Howes FN (2012) Plants for bees: a guide to the plants that benefit the bees of the british isles. IBRA, p 280
Kleijn D, Sutherland WJ (2003) How effective are European agri-environment schemes in conserving and promoting biodiversity? J Appl Ecol 40:947–969. doi:10.1111/j.1365-2664.2003.00868.x
Kleijn D, Berendse F, Smit R, Gilissen N (2001) Agri-environment schemes do not effectively protect biodiversity in Dutch agricultural landscapes. Nature 413:723–725. doi:10.1038/35099540
Knop E, Kleijn D, Herzog F, Schmid B (2006) Effectiveness of the Swiss agri-environment scheme in promoting biodiversity. J Appl Ecol 43:120–127. doi:10.1111/j.1365-2664.2005.01113.x
Lande R (1996) Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos 76:5–13. doi:10.2307/3545743
Le Féon V, Schermann-Legionnet A, Delettre Y et al (2010) Intensification of agriculture, landscape composition and wild bee communities: a large scale study in four European countries. Agric Ecosyst Environ 137:143–150. doi:10.1016/j.agee.2010.01.015
Leibold MA, McPeek MA (2006) Coexistence of the niche and neutral perspectives in community ecology. Ecology 87:1399–1410
Kuhlmann et al M (2013) Checklist of the western palaearctic bees (Hymenoptera: Apoidea: Anthophila). http://westpalbees.myspecies.info/. Accessed 10 Sep 2013
Magurran AE (2004) Measuring biological diversity. Blackwell Pub, Malden
Michener CD (2007) The Bees of the World, 2nd Revised Edition. Johns Hopkins University Press, Hopkins
Müller J, Goßner MM (2010) Three-dimensional partitioning of diversity informs state-wide strategies for the conservation of saproxylic beetles. Biol Conserv 143:625–633. doi:10.1016/j.biocon.2009.11.027
Munyuli MBT, Nyeko P, Potts S et al (2013) Patterns of bee diversity in mosaic agricultural landscapes of central Uganda: implication of pollination services conservation for food security. J Insect Conserv 17:79–93. doi:10.1007/s10841-012-9488-x
New TR (1999) Limits to species focusing in insect conservation. Ann Entomol Soc Am 92:853–860
Öckinger E, Smith HG (2007) Semi-natural grasslands as population sources for pollinating insects in agricultural landscapes. J Appl Ecol 44:50–59. doi:10.1111/j.1365-2664.2006.01250.x
Oertli S, Mueller A, Dorn S (2005) Ecological and seasonal patterns in the diversity of a species-rich bee assemblage (Hymenoptera: Apoidea: Apiformes). Euro J Entomol 102:53–63
Oksanen J, Blanchet FG, Kindt R et al (2011) Vegan: community ecology package. Blackwell Publishing, Oxford
Palmer MW (1995) How should one count species? Natural Areas Journal 15:124–135
Potts SG, Vulliamy B, Dafni A et al (2003) Linking bees and flowers: How do floral communities structure pollinator communities? Ecology 84:2628–2642
Potts SG, Vulliamy B, Roberts S et al (2005) Role of nesting resources in organising diverse bee communities in a Mediterranean landscape. Ecological Entomol 30:78–85. doi:10.1111/j.0307-6946.2005.00662.x
Potts S, Roberts S, Dean R et al (2010a) Declines of managed honey bees and beekeepers in Europe. J Apic Res 49:15. doi:10.3896/IBRA.1.49.1.02
Potts SG, Biesmeijer JC, Kremen C et al (2010b) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353. doi:10.1016/j.tree.2010.01.007
Pouvreau A (2004) Les insectes pollinisateurs. Delachaux et Niestlé, Paris
Ranta E, Lundberg H (1980) Resource partitioning in bumblebees: the significance of differences in proboscis length. Oikos 35:298–302. doi:10.2307/3544643
Rollin O, Bretagnolle V, Decourtye A et al (2013) Differences of floral resource use between honey bees and wild bees in an intensive farming system. Agric Ecosyst Environ 179:78–86. doi:10.1016/j.agee.2013.07.007
Roubik DW (2001) Ups and downs in pollinator populations: When is there a decline? Conserv Ecol 5. URL: http://www.ecologyandsociety.org/vol5/iss1/art2/
Scheiner SM (2003) Six types of species-area curves. Glob Ecol Biogeogr 12:441–447. doi:10.1046/j.1466-822X.2003.00061.x
Steffan-Dewenter I, Tscharntke T (2001) Succession of bee communities on fallows. Ecography 24:83–93. doi:10.1034/j.1600-0587.2001.240110.x
Summerville KS, Crist TO (2005) Temporal patterns of species accumulation in a survey of Lepidoptera in a beech-maple forest. Biodivers Conserv 14:3393–3406. doi:10.1007/s10531-004-0546-x
Tylianakis JM, Klein A-M, Tscharntke T (2005) Spatiotemporal variation in the diversity of Hymenoptera across a tropical habitat gradient. Ecology 86:3296–3302. doi:10.1890/05-0371
VanEngelsdorp D, Hayes J, Underwood RM, Pettis J (2008) A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. PLoS ONE 3:e4071. doi:10.1371/journal.pone.0004071
Walther BA, Morand S (1998) Comparative performance of species richness estimation methods. Parasitology 116:395–405
Westphal C, Steffan-Dewenter I, Tscharntke T (2003) Mass flowering crops enhance pollinator densities at a landscape scale. Ecol Lett 6:961–965. doi:10.1046/j.1461-0248.2003.00523.x
Westphal C, Bommarco R, Carré G et al (2008) Measuring bee diversity in different european habitats and biogeographical regions. Ecol Monogr 78:653–671. doi:10.1890/07-1292.1
Westrich P (1989) Die Wildbienen Baden-Württemburgs: Spezieller Teil—Die Gattungen und Arten. Eugen Ulmer, Stuttgart
Williams NM, Minckley RL, Silveira FA (2001) Variation in native bee faunas and its implications for detecting community changes. Conserv Ecol 5. URL: http://www.consecol.org/vol5/iss1/art7/
Zurbuchen A, Cheesman S, Klaiber J et al (2010a) Long foraging distances impose high costs on offspring production in solitary bees. J Anim Ecol 79:674–681. doi:10.1111/j.1365-2656.2010.01675.x
Zurbuchen A, Landert L, Klaiber J et al (2010b) Maximum foraging ranges in solitary bees: only few individuals have the capability to cover long foraging distances. Biol Conserv 143:669–676. doi:10.1016/j.biocon.2009.12.003
Acknowledgments
Special thanks go to J. Knapp and J. Osborne for thorough text correction and improvement. B. Vaissière provided expert support at all stages of the study, including the design and conception of the survey and the management of specimen collection and identification. We are also grateful to J. Aptel, M. Chabirand, A. Haefflinger, C. Maffre and C. Toulet for field assistance; H. Dathe, E. Dufrêne, R. Fonfria, D. Genoud, M. Kuhlmann, G. Le Goff, D. Michez, A. Pauly, S. Risch for bee identification to specie level; F. Herzog, D. Michez and I. Dajoz for helpful comments on earlier versions of the manuscript. We also thank the farmers of the study area for allowing us to carry out surveys in their fields. This work was funded by the French Ministry of Agriculture (POLINOV, CASDAR Program no 9035) and an ANRT CIFRE Ph.D. grant allocated to OR. This paper is dedicated in memoriam of Robert Fonfria.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jens Wolfgang Dauber.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rollin, O., Bretagnolle, V., Fortel, L. et al. Habitat, spatial and temporal drivers of diversity patterns in a wild bee assemblage. Biodivers Conserv 24, 1195–1214 (2015). https://doi.org/10.1007/s10531-014-0852-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-014-0852-x