Abstract
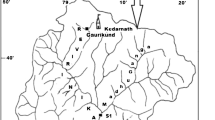
Use of diatoms in monitoring water quality is well acknowledged in developed countries, but only recently has the assessment started gaining importance in developing countries. Diatoms can be obtained from natural and artificial substrates. Appreciating the differences and similarities of diatom assemblages on both substrates may contribute to a better understanding and standardization particularly during monitoring of water quality. During this study we assessed diatom assemblages, biodiversity and trophic indices in relation to water quality along the Nairobi River. Fifteen sites were sampled in September 2000 during the dry season. Diatoms were collected from natural substrates (stones, pebbles) and artificial substrates (100% acrylic wool). On artificial and natural substrates, a total of 190 and 151 taxa were found, respectively, the majority of these taxa (80%) have cosmopolitan distribution and are also widespread throughout tropical African. Species composition changed downstream, five taxa dominated upper and mid stream sites whereas lower stream sites were dominated by one or two taxa. Species richness, diversity, dominance and evenness were positively correlated with NO3, O2 and altitude but decreased markedly downstream with a simultaneous increase in total dissolved solids, alkalinity, chemical oxygen demand and PO4. Ordination and classification (CANOCO and TWINSPAN) showed that diatom assemblages in the Nairobi River responded strongly to water quality changes with respect to concentrations of NO3, NO2, total dissolved solids and temperature. Taxa common at less impacted upstream sites included Gomphonema gracilis, Anomoeoneis brachysira and Fragilaria biceps; while common taxa at midstream sites with agricultural catchments were Gomphonema parvulum, Navicula cryptocephala, N. schroeteri, N. bryophila, N. halophila, Nitzschia linearis var. linearis and Cymbella silesica. Achnanthes minutissima var. saprophila, Gomphonema angustum, Navicula subminuscula, N. arvensis, Nitzschia palea and N. umbonata were most common at urban sites, which were polluted by residential and industrial effluents. Trophic diatom indices suggested that water quality was poor at most sites in the Nairobi River. Most sites along the river had low Generic Diatom Index values, GDI (<12) and high Trophic Diatom Index values, TDI 73–78 (median = 76) and 75–84 (median = 77) for artificial and natural substrates, respectively. This study showed that diatoms' response on natural and artificial substrates were similar and reflected environmental conditions correctly.
Similar content being viewed by others
References
Aboal M., Puig M.A. and Soler G. (1996). Diatom assemblages in some Mediterranean temporary streams in south-eastern Spain. Arch Hydrobiol. 136(4): 509–527
Acs E. and Kiss K.T. (1991). Investigation of periphytic algae in the Danube at God (1669 river kmHungary). Algol. Stud. 62: 47–67
APHA (American Public Health Association). (1995). Standards methods for the examination of water and wastewater19th ed. Port City Press, Baltimore, Maryland, USA
Biggs B.J.F. (1996). Patterns of benthic algae in streams. In: Stevenson, R.J., Bothwell, M.L. and Lowe, R.L. (eds) Algal Ecology–Freshwater Benthic Ecosystems, pp 31–56. Academic Press, California
Cattaneo A. and Amireault M.C. (1992). How artificial are artificial substrates for periphyton?. J. North Am. Benthol. Soc. 11: 244–256
Connell J.H. (1978). Diversity in tropical rain forests and coral reefs. Science 199: 1302–1310
Cronk J. and Mitsch W.J. (1994). Periphyton productivity on artificial and natural surfaces in constructed freshwater wetlands under different hydrologic regimes. Aquat. Bot. 48: 325–341
Dural B., Aysel V., Lok A. and Guner H. (1997). Benthic algal flora of the natural and artificial substrates of Hekim Island (IzmirTurkey). Algol. Stud. 85: 31–48
EU (European Union) 2000. Directive of the European Parliament and of the council 2000/60/EC establishing a framework for community action in the field of water policy. European Union. The European Parliament. The Council. PE-CONS 3639/1/00 REV 1 EN.
Fabricius A.L.M., Maidana N., Gomez M.N. and Sabater S. (2003). Distribution pattern of benthic diatoms in a Pampean river exposed to seasonal floods: the Cuarto River (Argentina). Biodivers. Conserv. 12(12): 2443–2454
Gasse F. 1986. East African Diatoms: Taxonomy, Ecological distribution. Berlin, Stuttgart, Germany
Germain H. (1981). Flore Diatomées. Boubée, Paris
HABITAT (United Nations Centre for Human Settlements). (2002). Human settlements basic statistics. Nairobi, Kenya
Hach Company. (1996). DR/2000 Spectrophotometer Procedures Manual. Loveland, Colorado, USA
Harding J.S., Young R.G., Hayes J.W., Shearer K.A. and Stark J.D. (1999). Changes in agricultural intensity and river health along a river continuum. Freshwater Biol. 42: 345–357
Hendey H.I. (1974). Permanganate method for cleaning freshly gathered diatoms. Microscopy 32: 423–426
Hill M.O. (1973). Diversity and evenness: a unifying notation and its consequences. Ecology 54: 427–432
Hillebrand H. and Sommer U. (2000). Diversity of benthic microalgae in response to colonization time and eutrophication. Aquat. Bot. 67: 221–236
Izsak C.A., Price R.G., Hardy J.T. and Basson P.W. (2002). Biodiversity of periphyton (diatoms) and echinoderms around a refinery effluentand possible associations with stability. Aquat. Ecosyst. Health Manage. 5: 61–70
Jeffries M. and Mills D. (1990). Freshwater Ecology: Principles and Application. Belhaven Press, London and New York
Jüttner I., Rothfritz H. and Ormerod S.J. (1996). Diatoms as indicators of rivers water quality in the Nepalese Middle Hills with consideration of the effects of habitats-specific sampling. Freshwater Biol. 36: 475–486
Jüttner I., Sharma S., Dahal B.M., Ormerod S.J., Chimonides P.J. and Cox E.J. (2003). Diatoms as indicators of stream quality in the Kathmandu Valley and Middle Hills of Nepal and India. Freshwater Biol. 48: 2065–2084
Kelly M. (2000). Identification of common benthic diatoms in rivers. Field Stud. 9: 583–700
Kelly M.G., Cazaubon A., Coring E., Dell'Uomo A., Ector L., Goldsmith B., Guasch H., Hrlimann J., Jarman A., Kawecka B., Kwandrans J., Laugaste R., Lindstrøm E.-A., Leitao M., Marvan P., Padisák J., Pipp E., Prygiel J., Rott E., Sabater S., Vizinet J. and Dam H. (1998). Recommendations for the routine sampling of diatoms for water quality assessments in Europe. J. Appl. Phycol. 10: 215–224
Kinyua A.M. and Pacini N. (1991). The impact of pollution on the ecology of the Nairobi-Athi River system in Kenya. J. Biochem. Phys. 1(1): 5–7
Köster D. and Hübener T. (2001). Application of diatom Indices in a planted ditch constructed for tertiary sewage treatment in Schwaan, Germany. Int. Rev. gesamten Hydrobiol. 86: 241–252
Krammer K. and Lange-Bertalot H. (1986–1991). Bacillariophyceae 1. Teil: Naviculaceace; 2. Teil: Bacillariophyceae, Epithemiaceae, Surirellaceae Bacillariophyceae; 3. Teil: Centrales, Fragilariaceae, Eunotiaceae; 4. Teil: Achnanthaceae, Naviculaceace, Gomphonema. In: Susswasserflora von, Mitteleuropa, Ettl, H., Gerloff, J., Heynig, H. and Mollenhauer, D. (eds) 2/1 I-XIX +, pp 1–876. Gustav Fischer-Verlag, Stuttgart
Leland H.V. and Porter S.D. (2000). Distribution of benthic algae in the upper Illinois River basin in relation to geology and land use. Freshwater Biol. 44: 279–301
Lobo E.A., Katoh K. and Aruga Y. (1995). Response of epilithic diatom assemblages to water pollution in rivers in the Tokyo Metropolitan areaJapan. Freshwater Biol. 34: 191–204
Lowe R.L. and Gale W.F. (1980). Monitoring river periphyton with artificial benthic substrates. Hydrobilogia 69(3): 235–244
Lowe R.L., Guckert J.B., Belanger S.E., Davidson D.H. and Johnson D.W. (1996). An evaluation of periphyton community structure and function on tile and cobble substrates in experimental stream mesocosms. Hydrobiologia 328: 135–146
McCormick P.V. and Stevenson R.J. (1998). Periphyton as tool for ecological assessment and management in the Florida Everglades. J. Phycol. 34: 726–733
McCune B. and Mefford M.J. (1999). Multivariate Analysis of Ecological Data, Version 4.0. Oregon, USA
Ndaruga A.M., Ndiritu G.G., Gichuki N.N. and Wamicha W.N. (2004). Impact of water quality on macroinvertebrates assemblages along a tropical stream in Kenya. Afr. J. Ecol. 42: 208–216
Ndiritu G.G., Gichuki N.N., Kaur P. and Triest L. (2003). Characterization of environmental gradients using physico-chemical measurements and diatom densities in Nairobi River, Kenya. Aquat. Ecosyst. Health Manage. 6(3): 343–354
O'Farrell I., Vinocur A. and Izaguirre I. (1996). Phytoplankton ecology of the lower Paraná River (Argentina). Arch. Hydrobiol. Suppl. 115: 75–89
Ometo J.P.H.B., Martinelli L.A., Ballester M.V., Gessner A., Krusche A.V., Victoria R.L. and Williams M. (2000). Effects of land use on water chemistry and macroinvertebrates in two streams of the Piracicaba River basin, southeast Brazil. Freshwater Biol. 44: 327–337
Patrick R. (1973). Use of algaeespecially diatoms, in assessment of water quality. In: (eds) Biological Methods for the Assessment of Water Quality, pp 76–95. America Society for Testing and Materials, Philadelphia, PA
Pielou E.C. (1975). Ecological Diversity. Wiley-Interscience, New York
Potapova M.G. and Charles D.F. (2002). Benthic diatoms in USA rivers: distributions along spatial and environmental gradients. J. Biogeogr. 29: 167–187
Raschke R.L. (1993). Diatom (Bacillariophyta) community response to phosphorus in Everglades National Park, USA. J. Phycol. 32: 48–58
Rott E. 1991. Methodological aspects and perspectives in the use of periphyton for monitoring and protecting rivers. In: Whitton B.A., Rott E. and Friedrich G. (eds), Use of algae for monitoring rivers. Austria, pp. 9–16
Rott E. and Pfister P. (1988). Natural epilithic algal communities in fast-flowing mountain streams and rivers and some man-induced changes. Verhandlungen Internationale Vereinigung für Theoretische und angewandte Limonologie 23: 1320–1324
Rumeau A. and Coste M. (1988). Initiation a la systématique des Diatomées d'eau douce pour I'utilisation pratique d'un indice diatomique générique. Bulletin Francais Pêche Piscuiculture 309: 1–69
Schoeman F.R. (1976). Diatom indicator groups in the assessment of water quality in the Jukskei-Crocodile River System (Transvaal, Republic of South Africa). J. Limnol. Soc. South Afr. 2: 21–24
Schöenfelder I., Gelbrecht J., Schöenfelder J. and Steinberg C.E.W. (2001). Relationship between littoral diatoms and their chemical environment in Northeastern German lakes and rivers. J. Phycol. 38: 66–82
Sims P.A., Barber H.G., Carter J.R. and Hartley B. (1996). Atlas of British Diatoms. Biopress, England
Snoeijs P.J.M (1991). Monitoring pollution effects by diatom community composition: A comparison of sampling methods. Arch. Hydrobiol. 121(4): 497–510
StatSoft Inc. (1996). STATISTICA for Windows. Tulsa, OK, USA
Stevenson R.J. and Pan Y. 1999. Assessing environmental conditions in rivers and streams with diatoms. In: Stoermer E. and Smol J.P. (eds), The diatoms: applications for the environmental and earth sciences. Cambridge University Press, pp. 11–41.
Stevenson R.J. (1984). Epilithic and epipelic diatoms in the Sandusky Riverwith emphasis on species diversity and water quality. Hydrobiologia 114: 161–175
Stevenson R.J. (1997). Scale-dependent causal framework and the consequences of benthic algal heterogeneity. J. North Am. Benthol. Soc. 16: 248–262
Stoermer E. and Smol J.P. 1999. The diatoms: applications for the environmental and earth sciences. Cambridge University Press.
ter Braak J.F. and Smilauer P. (1999). Canoco for windows, version 4.02. Wageningen, The Netherlands
UNEP (United Nations Environment Programme). (2001). The Nairobi River Project (ROA). Nairobi, Kenya
van Dam H. (1982). On the use of measures of structure and diversity in applied diatom ecology. Nova Hedwigia 73: 97–115
Mertens A., Sinkeldam J. and Dam H. (1994). A coded checklist and ecological values of freshwater diatoms from Netherlands. Neth. J. Aquat. Ecol. 28(1): 117–133
Vannote R.L., Minshall G.W., Cummis K.W., Sedell J.R. and Cushing C. (1980). The river continuum concept 1. Can. J. Fish. Aquat. Sci. 37: 130–137
Ward J.V. (1998). Riverine landscapes: biodiversity patterns, disturbance regimes and aquatic conservation. Biol. Conserv. 83(3): 269–278
Waters G. and Odero J. (1986). Geography of Kenya and East African Regions. Macmillan Publishers, London
Whitton B.A. and Rott E. (1996). Proceedings of an International Symposium on Use of Algae for Monitoring Rivers. 17–19 September 1995. Austrian Ministry of Science Traffic and Arts, Innsbruck, Austria
Whitton B.A. and Kelly M.G. (1995). Use of algae and other plants for monitoring rivers. Aust. J. Ecol. 20: 45–56
Wood E.D., Armstrong F.A.J. and Richards F.A. (1967). Determination of nitrates in seawater by cadmium-copper reduction to nitrate. J. Marine Biol. 47: 23–30
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ndiritu, G.G., Gichuki, N.N. & Triest, L. Distribution of Epilithic Diatoms in Response to Environmental Conditions in an Urban Tropical Stream, Central Kenya. Biodivers Conserv 15, 3267–3293 (2006). https://doi.org/10.1007/s10531-005-0600-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-005-0600-3