Abstract
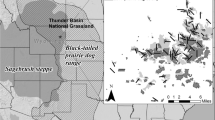
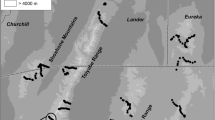
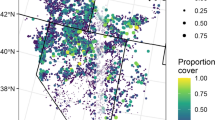
Invasions by nonnative plants can alter the abundance of native animals, yet we know little about the mechanisms driving these changes. Shifts in vegetation characteristics resulting from nonnative plants can alter availability of food resources, predation risk, and foraging efficiency (both the access to and ability to find food), each providing a potential mechanism for documented changes in animal communities and populations in invaded systems. Cheatgrass (Bromus tectorum) is a nonnative grass that invades sagebrush steppe, resulting in declines in some small mammal populations. We examined whether changes in structural characteristics associated with cheatgrass invasion could alter foraging by small mammals, providing a potential mechanism for documented population declines. We quantified differences in vegetation structure between native and cheatgrass-invaded sagebrush steppe, then experimentally added artificial structure in native areas to simulate these differences. We placed grain at foraging stations and measured the amount removed by small mammals nightly. Adding litter at depths approximating invasion by cheatgrass reduced the average amount of grain removed in 2 of 3 study areas, but increasing stem density did not. Based on this experiment, the deeper litter created by cheatgrass invasion may increase costs to small mammals by decreasing foraging efficiency and access to existing food resources, which may explain population-level declines in small mammals documented in other studies. By isolating and identifying which structural attributes of cheatgrass invasion are most problematic for small mammals, land managers may be able to design treatments to efficiently mitigate impacts and restore invaded ecosystems.




Similar content being viewed by others
References
Arnan X, Rodrigo A, Retana J (2007) Uncoupling the effects of shade and food resources of vegetation on Mediterranean ants: an experimental approach at the community level. Ecography 30:161–172
Bachen DA (2014) Cheatgrass invasion of sagebrush steppe: Impacts of vegetation structure on small mammals. Thesis, Montana State University
Bradley BA (2009) Regional analysis of the impacts of climate change on cheatgrass invasion shows potential risk and opportunity. Glob Change Biol 15:196–208
Brown JS (1988) Patch use as an indicator of habitat preference, predation risk, and competition. Behav Ecol Sociobiol 22:37–47
Ceradini JP, Chalfoun AD (2017) When perception reflects reality: non-native grass invasion alters small mammal risk landscapes and survival. Ecol Evol 7:1823–1835
Cody ML (1981) Habitat selection in birds: the role of vegetation structure, competitors and productivity. Bioscience 31:107–113
Connolly BM, Pearson DE, Mack RN (2014) Granivory of invasive, naturalized, and native plants in communities differentially susceptible to invasion. Ecology 95:1759–1769
Crawford JA, Olson RA, West NE, Mosley JC, Schroder MA, Whitson TD, Miller RF, Gregg MA, Boyd CS (2004) Ecology and management of sage-grouse and sage-grouse habitat. Rangel Ecol Manag 57:2–9
Crist TO, Guertin DS, Wiens JA, Milne BT (1992) Animal movement in heterogeneous landscapes: an experiment with Eleodes beetles in shortgrass prairie. Funct Ecol 6:536–544
Daubenmire R (1959) A canopy-coverage method of vegetation analysis. Northwest Sci 33:43–64
Dutra HP, Barnett K, Reinhardt JR, Marquis RJ, Orrock JL (2011) Invasive plant species alters consumer behavior by providing refuge from predation. Oecologia 166:649–657
Freeman ED, Sharp TR, Larson RT, Knight RN, Slater SJ, McMillan BR (2014) Negative effects of an exotic grass invasion on small mammal communities. PLoS ONE 9:e108843. https://doi.org/10.1371/journal.pone.0108843
Gano KA, Rickard WH (1982) Small mammals of a bitterbrush-cheatgrass community. Northwest Sci 56:1–8
Garden JG, McAlpine CA, Possingham HP, Jones DN (2007) Habitat structure is more important than vegetation composition for local-level management of native terrestrial reptile and small mammal species living in urban remnants: a case study from Brisbane, Australia. Austral Ecol 32:669–685
Gregg MA, Crawford JA (2010) Survival of greater sage-grouse chicks and broods in the northern Great Basin. J Wildl Manag 73:904–913
Guiden PW, Orrock JL (2017) Invasive exotic shrub modifies a classic animal-habitat relationship and alters patterns of vertebrate seed predation. Ecology 98:321–327
Hall LK (2012) Effect of cheatgrass on abundance of the North American deer mouse (Peromyscus maniculatus). Southwest Nat 57:166–169
Johnson MD, De León YL (2015) Effect of an invasive plant and moonlight on rodent foraging behavior in a coastal dune ecosystem. PLoS ONE 10(2):e0117903
Kelrick MI, MacMahon JA, Parmenter RR, Sisson DV (1986) Native seed preferences of shrub-steppe rodents, birds and ants: the relationships of seed attributes and seed use. Oecologia 68:327–337
Knapp PA (1996) Cheatgrass (Bromus tectorum L.) dominance in the Great Basin Desert: history, persistence, and influences to human activities. Glob Environ Change 6:37–52
Larrison EJ, Johnson DR (1973) Density changes and habitat affinities of rodents of shadescale and sagebrush associations. Great Basin Nat 33:255–264
Lesica P, Lavin MT, Stickney PF (2012) Manual of Montana Vascular Plants. BRIT Press, Fort Worth, p 771
Levine JM, Vilà M, D’Antonio CM, Dukes JS, Grigulis K, Lavorel S (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc R Soc Lond Ser B Biol Sci 270:775–781
Litt AR, Pearson DE (2013) Non-native plants and wildlife in the intermountain west. Wildl Soc Bull 37(3):517–526
Litt AR, Cord EE, Fulbright TE, Schuster GL (2014) Effects of invasive plants on arthropods. Conserv Biol 28:1532–1549
Lucero JE, Allen PS, McMillan BR (2015) Increased primary production from an exotic invader does not subsidize native rodents. PLoS ONE 10:e0131564
Mattos KJ, Orrock JL (2010) Behavioral consequences of plant invasion: an invasive plant alters rodent antipredator behavior. Behav Ecol 21:556–561
Mattos KJ, Orrock JL, Watling JI (2013) Rodent granivores generate context-specific seed removal in invaded and uninvaded habitats. Am Midland Nat 169:168–178
Mitchell WA, Abramsky Z, Kotler BP, Pinshow B, Brown JS (1990) The effect of competition on foraging activity in desert rodents: theory and experiments. Ecology 71:844–854
Orrock JL, Danielson BJ, Brinkerhoff RJ (2004) Rodent foraging is affected by indirect, but not by direct, cues of predation risk. Behav Ecol 15:433–437
Ortega YK, McKelvey KS, Six DL (2006) Invasion of an exotic forb impacts reproductive success and site fidelity of a migratory songbird. Oecologia 149:340–351
Ostoja SM, Schupp EW (2009) Conversion of sagebrush shrublands to exotic annual grasslands negatively impacts small mammal communities. Divers Distrib 15:863–870
Pearson DE (2009) Invasive plant architecture alters trophic interactions by changing predator abundance and behavior. Oecologia 159:549–558
Pearson DE (2010) Trait-and density-mediated indirect interactions initiated by an exotic invasive plant autogenic ecosystem engineer. Am Nat 176:394–403
Pearson DE, Fletcher RJ (2008) Mitigating exotic impacts: restoring deer mouse populations elevated by an exotic food subsidy. Ecol Appl 18:321–334
Pyke GH (1984) Optimal foraging theory: a critical review. Annu Rev Ecol Syst 15:523–575
Pyšek P, Jarošík V, Hulme PE, Pergl J, Hejda M, Schaffner U, Vilà M (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species' traits and environment. Glob Change Biol 18:1725–1737
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org. Accessed 25 Oct 2015
Rickard WH (1970) Ground dwelling beetles in burned and unburned vegetation. J Range Manag 23:293–294
Rieder JP, Newbold TS, Ostoja SM (2010) Structural changes in vegetation coincident with annual grass invasion negatively impacts sprint velocity of small vertebrates. Biol Invasions 12:2429–2439
Rosenzweig ML (1973) Habitat selection experiments with a pair of coexisting heteromyid rodent species. Ecology 54:111–117
Simonetti JA (1989) Microhabitat use by small mammals in central Chile. Oikos 56:309–318
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. WH. Freeman and Co., San Francisco
Thompson SD (1982) Structure and species composition of desert heteromyid rodent species assemblages: effects of a simple habitat manipulation. Ecology 63:1313–1321
Vásquez RA, Ebensperger LA, Bozinovic F (2002) The influence of habitat on travel speed, intermittent locomotion, and vigilance in a diurnal rodent. Behav Ecol 13:182–187
Verdolin JL (2006) Meta-analysis of foraging and predation risk trade-offs in terrestrial systems. Behav Ecol Sociobiol 60:457–464
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708
Vitousek PM, D’Antonio CM, Loope LL, Westbrooks R (1996) Biological invasions as global change. Am Sci 84:468–478
Ydenberg RC, Welham CVJ, Schmid-Hempel R, Schmid-Hempel P, Beauchamp G (1994) Time and energy constraints and the relationships between currencies in foraging theory. Behav Ecol 5:28–34
Young JA, Evans RA (1973) Downy brome: intruder in the plant succession of big sagebrush communities in the Great Basin. J Range Manag 26:410–415
Acknowledgements
We thank Dr. Megan Higgs for her assistance throughout this project. Comments from Dr. Higgs and several anonymous reviewers greatly improved this manuscript. We also appreciate financial and logistical support from the Department of Ecology at Montana State University, Montana Fish, Wildlife, and Parks, the Montana Chapter of The Wildlife Society, the Montana Institute on Ecosystems, and the Bureau of Land Management.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bachen, D.A., Litt, A.R. & Gower, C.N. Simulating cheatgrass (Bromus tectorum) invasion decreases access to food resources for small mammals in sagebrush steppe. Biol Invasions 20, 2301–2311 (2018). https://doi.org/10.1007/s10530-018-1701-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-018-1701-8