Abstract
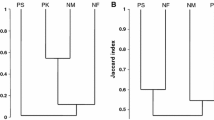
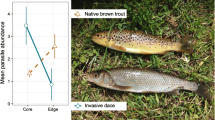
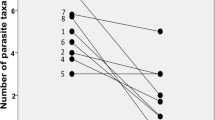
There is increasing evidence that parasitism represents an unpredictable dimension of the ecological impacts of biological invasions. In addition to the risk of exotic pathogen transmission, other mechanisms such as parasite-release, could contribute to shaping the relationship between introduced species and native communities. In this study, we used the Eurasian round goby (Neogobius menalostomus) in the Great Lakes-St. Lawrence River ecosystem to further explore these ideas. As predicted by the parasite-release hypothesis, recently established populations of round goby were parasitized by a depauperate community of generalist helminths (8 taxa), all commonly found in the St. Lawrence River. In comparison, two native species, the logperch (Percina caprodes) and spottail shiner (Notropis hudsonius), were the hosts of 25 and 24 taxa respectively. Round gobies from each of 3 sampled localities were also less heavily infected than both indigenous species. This is in contrast to what is observed in round goby’s native range where the species is often the most parasitized among gobid competitors. This relative difference in parasite pressure could enhance its competitiveness in the introduced range. However, our study of an older population of round goby in Lake St. Clair suggests that this advantage over native species could be of short duration. Within 15 years, the parasite abundance and richness in the round goby has more than doubled whereas the number of parasite species per fish has increased to levels of those typical of fish indigenous to the St. Lawrence-Great Lakes watershed.






Similar content being viewed by others
References
Amin OM (2002) Revision of Neoechinorhynchus Stiles and Hassall, 1905 (Acanthocephala: Neoechinorhychidae) with keys to 88 species in two subgenera. Syst Parasitol 53:1–18
Amin OM, Muzzall PM (2009) Redescription of Neoechinorhynchus tenellus (Acanthocephala: Neoechinorhynchidae) from Esox lucius (Esocidae) and Sander vitreus (Percidae), among other Percid and Centrarchid fish, in Michigan, U.S.A. Comp Parasitol 76(1):44–50
Anderson RM, May RM (1986) The invasion, persistence and spread of infectious diseases with animal and plant communities. Phil Trans R Soc Lond B Biol Sci 314:533–570
Arai HP (1989) Acanthocephala. In: Margolis L, Kabata Z (ed) Guide to the parasites of fishes of Canada. Part III. Can Spec Publ Fish Aquat Sci 107:1–90
Balshine S, Verma A, Chant V, Theysmeyer T (2005) Competitive interactions between round gobies and logperch. J Great Lakes Res 31:68–77
Bergstrom MA, Mensinger AF (2009) Interspecific resource competition between the invasive round goby and three native species: logperch, slimy sculpin, and spoonhead sculpin. Trans Am Fish Soc 138:1009–1017
Blakeslee AMH, Keogh CL, Byers JE, Kuris AM, Lafferty KD, Torkin ME (2009) Differential escape from parasites by two competing introduced crabs. Marine Ecol Prog Ser 393:83–96
Blaustein AR, Kuris AM, Alio JJ (1983) Pest and parasite species richness problems. Am Nat 122:556–566
Brassard P, Rau ME, Curtis MA (1982) Infection dynamics of Diplostomum spathaceum cercariae and parasite-induced mortality of fish hosts. Parasitology 85:489–493
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83(4):575–583
Camp JW, Blaney LM, Barnes DK (1999) Helminths of the round goby, Neogobius melanostomus (Perciformes: Gobiidae), from Southern Lake Michigan, Indiana. J Helminthol Soc Wash 66(1):70–72
Campton DE, Busack CA (1989) Simple procedure for decapsulating and hatching cysts of brine shrimp (Artemia spp.). Prog Fish-Cult 51:176–179
Charlebois PM, Marsden JE, Goettel RG, Wolfe RK, Jude DJ, Rudnika S (1997) The round goby, Neogobius melanostomus (Pallas), a review of European and North American literature. Illinois-Indiana Sea Grant Program and Illinois Natural History Survey, Illinois
Colautti R, Ricciardi A, Grigorovitch IA (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733
Conover W (1999) Practical non-parametric statistics, 3rd edn. Wiley, New York
Cornell HV, Hawkins BA (1993) Accumulation of native parasitoid species on introduced herbivores: a comparison of “hosts-as-natives” and “hosts-as-invaders”. American Naturalist 141:847–865
Cunningham AA, Daszak P, Rodriguez JP (2003) Pathogen pollution: defining a parasitological threat to biodiversity conservation. J Parasitol 89:S78–S83
Dermott R, Mitchell J, Murray I, Fera E (1993) Biomass and production of zebra mussels (Dreissena polymorpha) in shallow waters of northeastern Lake Erie. In: Nalepa TF, Schloesser DW (eds) Zebra mussels: biology, impacts and control. Lewis Publishers, Boca Raton, FL, pp 399–413
Dezfuli BS, Volponi S, Beltrami I, Poulin R (2002) Intra- and interspecific density-dependent effects on growth in helminth parasites of the cormorant, Phalacrocorax carbo sinensis. Parasitology 124(5):537–544
Dubs DOL, Corkum LD (1996) Behavioral interactions between round gobies (Neogobius melanostomus) and mottled sculpins (Cottus bairdi). J Great Lakes Res 22(4):838–844
Dunn AM (2009) Parasites and biological invasions. Adv Parasitol 68:161–184
Fredensborg BL, Poulin R (2005) Larval helminths in intermediate hosts: does competition early in life determine the fitness of adult parasites? Int J Parasitol 35(10):1061–1070
Gaudreau N (2005) Rapport sur la situation du dard de sable (Ammocrypta pellucida) au Québec. Ministère des Ressources naturelles et de la Faune du Québec, Secteur Faune Québec
Gibson DI (1996) Trematoda. In: Margolis L, Kabata Z (ed) Guide to the parasites of fishes of Canada. Part IV. Can Spec Publ Fish Aquat Sci 124:1–373
Griffiths RW (1993) Effects of zebra mussels (Dreissena polymorpha) on the benthic fauna of Lake St. Clair. In: Nalepa TF, Schloesser DW (eds) Zebra mussels: biology, impacts and control. Lewis Publishers, Boca Raton, pp 415–437
Guégan JF, Kennedy CR (1993) Maximum local helminth parasite community richness in British freshwater fish: a test of the colonization time hypothesis. Parasitology 106:91–100
Haynes JM (1997) Zebra mussels and benthic macroinvertebrate communities of southwestern Lake Ontario and selected tributaries: unexpected results? Great Lakes Res Rev 3(1):9–15
Haynes JM, Tisch NA, Mayer CM, Rhyne RS (2005) Benthic macroinvertebrate communities in southwester Lake Ontario following invasion of Dreissena, Echinogammarus : 1983 to 2000. J N Am Benthol Soc 24(10):148–167
Hoffman GL (1999) Parasites of North American freshwater fishes, 2nd edn. Comstock Publishing Associates, Ithaca
Horwitz P, Wilcox BA (2005) Parasites, ecosystems and sustainability: an ecological and complex systems perspective. Int J Parasitol 35(7):725–732
Hudson P, Dobson AP, Lafferty KD (2006) Is a healthy ecosystem one that is rich in parasites? Trends Ecol Evol 21(7):381–385
Janovy J Jr, Snyder SD, Clopton RE (1997) Evolutionary constraints on population structure: the parasites of Fundulus zebrinus (Pisces: Cyprinodontidae) in the South Platte River of Nebraska. J Parasitol 83:584–592
Janssen J, Jude DJ (2001) Recruitment failure of mottled sculpin Cottus bairdi in Calumet Harbor, southern Lake Michigan, induced by the newly introduced round goby Neogobius melanostomus. J Great Lakes Res 27(3):319–328
Kelly DW, Paterson RA, Townsend CR, Poulin R, Tompkins DM (2009) Parasite spillback: A neglected concept in invasion ecology? Ecology 90(8):2047–2056
Kopp K, Jokela J (2007) Resistant invaders can convey benefits to native species. Oikos 116:295–301
Krakau M, Thieltges DW, Reise K (2006) Native parasites adopt introduced bivalves of the North Sea. Biol Invasions 8:919–925
Krause RJ, McLaughlin D, Marcogliese DJ (2010) Parasite fauna of Etheostoma nigrum (Percidae: Etheostomatinae) in localities of varying pollution stress in the St. Lawrence River, Quebec, Canada. Parasitol Res 107:285–294
Kvach Y (2002) Helminths of goby fish of the Hryhoryivsky estuary (Black sea, Ukraine). Vestnik Zoologii 36(3):71–76
Kvach Y (2005) A comparative analysis of helminth faunas and infection parameters of ten species of gobiid fishes (Actinopterygii: Gobiidae) from the north-western Black Sea. Acta Ichthyol Pisc 35(2):102–110
Kvach Y, Skóra KE (2006) Metazoan parasites of the invasive round goby Apollonia melanostoma (Neogobius melanostomus) (Pallas) (Gobiidae: Osteichthyes) in the Gulf of Gdansk, Blatic Sea, Poland: a comparison with the Black Sea. Parasitol Res 100:767–774
Kvach Y, Stepien CA (2008) Metazoan parasites of introduced round and tubenose gobies in the Great Lakes: support for the “enemy release hypothesis”. J Great Lakes Res 34:23–35
Lapointe M (1997) Rapport sur la situation du fouille-roche gris (Percina copelandi) au Québec. Ministère de l’Environnement et de la Faune, Direction de la faune et des habitats Québec
Lauer TE, Allen PJ, McComish TS (2004) Changes in mottled sculpin and johnny darter trawl catches after the appearance of round gobies in the Indiana waters of Lake Michigan. Trans Am Fish Soc 133:185–189
Liu H, Stiling P (2006) Testing the enemy release hypothesis: a review and meta-analysis. Biol Invasions 8:1535–1545
Lymbery AJ, Hassan M, Morgan DL, Beatty SJ, Doupé RG (2010) Parasites of native and exotic freshwater fishes in south-western Australia. J Fish Biol 76:1770–1785
MacLeod C, Paterson AM, Tompkins DM, Duncan RP (2010) Parasites lost–do invaders miss the boat or drown on arrival? Ecol Lett 13(4):516–527
Marcogliese DJ (2001) Implications of climate change for parasitism of animals in the aquatic environment. Can J Zool 79:1331–1352
Marcogliese DJ (2004) Parasites: small players with crucial roles in the ecological theatre. EcoHealth 1:151–164
Marcogliese DJ (2008) First report of the Asian fish tapeworm in the Great Lakes. J Great Lakes Res 34:566–569
Marcogliese DJ, Compagna S (1999) Diplostomatid eye flukes in young-of-the-year and forage fishes in the St. Lawrence River, Quebec. J Aquat Anim Health 11:275–282
Marcogliese DJ, Pietrock M (2011) Combined effects of parasites and contaminants on animal health: parasites do matter. Trends Parasitol 27:123–130
Marcogliese DJ, Gendron AD, Plante C, Fournier M, Cyr D (2006) Parasites of spottail shiners (Notropis hudsonius) in the St. Lawrence River: effects of municipal effluents and habitat. Can J Zool 84:1461–1481
Marr S, Mautz WJ, Hara AH (2008) Parasite loss and introduced species: a comparison of the parasites of the Puerto Rican tree frog (Eleutherodactylus coqui), in its native and introduced ranges. Biol Invasions 10:1289–1298
Moravec F (1994) Parasitic nematodes of freshwater fishes of Europe. Kluwer Academic Publishers, Dordrecht
Muzzall PM, Whelan G (2011) Parasites of fish from the Great Lakes: a synopsis and review of the literature, 1871–2010. Great Lakes Fish Comm Misc Publ 2011-01
Muzzall PM, Peebles CR, Thomas MV (1995) Parasites of the round goby, Neogobius melanostomus, and tubenose goby, Proterorhinus marmoratus (Perciformes: Gobiidae), from the St. Clair River and Lake St. Clair, Michigan. J Helminthol Soc Wash 62(2):226–228
Ondračkova M, Dávidová M, Pečinková M, Blažek R, Glnar M, Vlaová Z, Černý J (2005) Metazoan parasites of Neogobius fishes in the Slovak section of the River Danube. J Appl Ichthyol 21:345–349
Ondračkova M, Francova K, Dávidová M, Dávidová M, Polačik M, Jurajda P (2010) Condition status and parasite infection of Neogobius kessleri and N. melanostomus (Gobiidae) in their native and non-native area of distribution of the Danube River. Ecol Res 25:857–866
Özer A (2007) Metazoan parasite fauna of the round goby Neogobius melanostomus Pallas, 1811 (Perciformes: Gobiidae) collected from the Black Sea coast at Sinop, Turkey. J Nat Hist 41(9–12):483–492
Poulin R (2001) Another look at the richness of helminth communities in tropical freshwater fish. J Biogeogr 28(6):737–743
Pronin NM, Fleisher GW, Baldanova DR, Pronina SV (1997) Parasites of the recently established round goby (Neogobius melanostomus) and tubenose goby (Proterorhinus marmoratus) (Cottidae) from the St. Clair River and Lake St. Clair, Michigan, USA. Folia Parasitol 44:1–6
Reyjola Y, Brodeur P, Mailhot Y, Mingelbier M, Dumont P (2010) Do native predators feed on non-native prey? The case of round goby in a fluvial piscivorous fish assemblage. J Great Lakes Res 36(4):618–624
Ricciardi A, Neves RJ, Rasmussen JB (1998) Impending extinctions of North American freshwater mussels (Unionoida) following the zebra mussel (Dreissena polymorpha) invasion. J Anim Ecol 67:613–619
Ricker WE (1975) Computation and interpretation of biological statistics of fish populations. Bull Fish Res Board Can 191:1–382
Roche DG, Leung B, Mendoza Franco EF, Torchin ME (2010) Higher parasite richness, abundance and impact in native versus introduced cichlid fishes. Int J Parasitol 40:1525–1530
Rolbiecki L (2006) Parasites of the round goby, Neogobius melanostomus (Pallas, 1811), an invasive species in the Polish fauna of the Vistula Lagoon ecosystem. Oceanologia 48(4):545–561
Ross JL, Ivanova ES, Severns PM, Wilson MJ (2010) The role of parasite release in invasion of the USA by European slugs. Biol Invasions 12:603–610
Sasal P, Morand S, Guégan JF (1997) Determinants of parasite species richness in Mediterranean marine fishes. Mar Ecol Prog Ser 149:61–71
Shaw DJ, Dobson AP (1995) Patterns of macroparasite abundance and aggregation in wildlife populations: a quantitative review. Parasitology 111:S111–S133
Stables LH, Chappell JN (1986) Diplostomum spathaceum (Rud. 1819) effect of physical factors on the infection of rainbow trout (Salmo gairdneri) by cercariae. Parasitology 93:71–79
Taraschewski H (2006) Hosts and parasites as aliens. J Helminthol 80:99–128
Telfer S, Bown KJ, Seules R, Begon M, Hayden T, Birtles R (2005) Disruption of a host-parasite system following the introduction of an exotic host species. Parasitology 130:661–668
Tompkins DM, Dunn AM, Smith MJ, Telfer S (2010) Wildlife diseases: from individuals to ecosystems. J Anim Ecol 80(1):19–38
Torchin ME, Lafferty KD (2009) Escape from parasites. In: Rilov G, Crooks JA (eds) Biological invasions in marine ecosystems. Ecological studies 204. Springer, Berlin, pp 203–214
Torchin ME, Mitchell CE (2004) Parasites, pathogens, and invasions by plants and animals. Front Ecol Environ 2:183–190
Torchin ME, Lafferty KD, Kuris AM (2001) Release from parasites as natural enemies: increased performance of a globally introduced marine crab. Biol Invasions 3:333–345
Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM (2003) Introduced species and their missing parasites. Nature 421:628–630
Voth DR, Anderson LF, Kleinschuster SJ (1974) The influence of waterflow on brown trout parasites. Prog Fish-Cult 36:212
Walther BA, Cotgreave P, Price RD, Gregory RD, Clayton DH (1995) Sampling effort and parasite species richness. Parasitol Today 11(8):306–310
Whittington ID, Cribb BW, Hamwood TE, Halliday JA (2000) Host-specificity of monogenean (platyhelminth) parasites: a role for anterior adhesive areas? Int J Parasitol 30(3):305–320
Acknowledgments
We would like to thank Germain Brault, Michel Arsenault, Claude Lessard, Sophie Trépanier, Marie-Pier Ricard, Jacinthe Gosselin, Eugénie Schaff, Pierre-Olivier Benoit, Maxime Guérard, Jean-François Lafond, Maude Lachapelle, Hubert Désilets, Lila Gagnon-Brambilla, Roxane Pétel and Maxime Thibault for their help in the collection of gobies from the St. Lawrence River and/or examination of fish for parasites. Round gobies from Lake St. Clair were collected by staff from the Michigan Department of Natural Resources’ Lake St. Clair Fisheries Research Station during scheduled surveys conducted under Federal Aid Study F-81-R-9 Study 230488. We are thankful to Christopher Blanar for his contribution to parasite identification. Pierre Gagnon is acknowledged for mathematical and statistical advice. The insightful suggestions and comments of two anonymous referees significantly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gendron, A.D., Marcogliese, D.J. & Thomas, M. Invasive species are less parasitized than native competitors, but for how long? The case of the round goby in the Great Lakes-St. Lawrence Basin. Biol Invasions 14, 367–384 (2012). https://doi.org/10.1007/s10530-011-0083-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-0083-y