Abstract
Campylopus introflexus is an invasive moss in Europe and North America that is adapted to acidic and nutrient-poor sandy soils with sparse vegetation. In habitats like acidic coastal dunes (grey dunes) it can reach high densities, build dense carpets and modify habitat conditions. While the impact of the moss invasion on the vegetation is well analyzed, there is a lack of knowledge regarding possible effects on arthropods. In the present study we analyzed the impact of Campylopus introflexus on the ground-dwelling arthropods carabid beetles and spiders, as both taxa are known to be useful indicator taxa even on a small-scale level. In 2009 we compared species composition in a) invaded, moss-rich (C. introflexus) and b) native, lichen-rich (Cladonia spp.) acidic coastal dunes by using pitfall traps. A total of 1,846 carabid beetles (39 species) and 2,682 spiders (66 species) were caught. Species richness of both taxa and activity densities of spiders were lower in invaded sites. Species assemblages of carabids and spiders differed clearly between the two habitat types and single species were displaced by the moss encroachment. Phytophagous carabid beetles, web-building spiders and wolf spiders were more abundant in native, lichen-rich sites. Shifts in species composition can be explained by differences in the vegetation structure, microclimate conditions and most likely a reduced food supply in invaded sites. By forming dense carpets and covering large areas, the moss invasion strongly alters typical arthropod assemblages of endangered and protected (EU-directive) acidic coastal dunes.
Similar content being viewed by others
Introduction
Since the middle of the last century, the neophytic moss Campylopus introflexus (Heath Star Moss), originating from the southern hemisphere, has been an invasive species in Europe and North America (Frahm 1980; Richards 1963; van der Meulen et al. 1987; Hassel and Söderström 2005). The Heath Star Moss is adapted to acidic and nutrient-poor sandy soils with sparse vegetation (Pott 1995). In Europe C. introflexus is widespread in habitats like acidic coastal dunes, inland dunes, drift sands and heathlands where it can reach high densities, build dense carpets and therefore alter habitat conditions (Biermann and Daniëls 1997; Ellenberg 1996; Ketner-Oostra and Sykora 2008; Pott 1995). Moss carpets can hinder the germination of other plants and cryptogams due to decreased water supply by interception (Biermann and Daniëls 2001), the lack of space for settlement, and a limited dispersal and establishment of some native plant species resulting in permanent displacement of these affected species (Hasse 2005, 2007; Ketner-Oostra and Sykora 2004, 2008). Thus, plant and lichen diversity of these habitats is endangered by the moss encroachment (Ketner-Oostra and Sykora 2004).
While the impact of the moss invasion on the vegetation is well analyzed (e.g. Equihua and Usher 1993; van der Meulen et al. 1987; Biermann and Daniëls 1997; Hasse 2005, 2007; Ketner-Oostra and Sykora 2004, 2008), knowledge of effects on arthropods is lacking (apart from Vogels et al. 2005; Schirmel submitted). This is remarkable, given that acidic coastal dunes belong to open, dry and nutrient-poor habitat types (like inland dunes, heathlands, dry grasslands) that are protected by the EU Habitats Directive (Ssymank et al. 1998) and present important habitats for many thermo- and xerophilous invertebrates (e.g. Maes et al. 2006; Maes and Bonte 2006; Riksen et al. 2006). These ecosystems and their specialized fauna are mainly threatened by land-use changes (e.g. abandonment of traditional land use) and atmospheric deposition (de Vries et al. 1994; Muller et al. 1998; Ketner-Oostra and Sykora 2004; Remke et al. 2009). Moreover, the invasion of the invasive moss Campylopus introflexus might present a novel threat especially for acidic coastal and inland dunes (Riksen et al. 2006; Ketner-Oostra and Sykora 2004). Since the 1980s the exotic moss can be found on the isle of Hiddensee in the German Baltic Sea (Schubert pers. comm.) where it has mainly invaded lichen-rich acidic coastal dunes (grey dunes) (Remke pers. comm.). While lichens are very sensitive to trampling and grazing, C. introflexus has an effective vegetative propagation (shoot fragments) and grows quickly (Biermann and Daniëls 1997). The spreading of C. introflexus in the lichen-rich acidic coastal dunes on the island might therefore be favored through trampling by tourists and an increased grazing pressure since the reintroduction of sheep pasturing in 2004.
Carabid beetles (Coleoptera: Carabidae) and spiders (Araneae) are useful indicator taxa utilized to monitor shifts in habitat conditions in nearly all kinds of terrestrial ecosystems (e.g. for carabids: Mossakowski and Främbs 1993; Desender 1996; Buchholz et al. 2009; for spiders: Hsieh et al. 2003; Warui et al. 2005; Huber et al. 2007; Horvath et al. 2009). Both taxa are rich in both individuals and species, have high reproduction rates, include several stenotopic species and feature a well known ecology (Lövei and Sunderland 1996; Turin 2000; Irmler and Gürlich 2004; Entling et al. 2007; Negro et al. 2009). As shown in other studies, the invasion of plants can have a great effect on the indigenous carabid (e.g. Hansen et al. 2009; Topp et al. 2008; Gu et al. 2008) or spider fauna (e.g. Petillon et al. 2005; Bultmann and Dewitt 2008; Mgobozi et al. 2008). As described above, the invasion of C. introflexus could change the vegetation structure of native sites and therefore might alter microhabitat conditions. As both carabid beetles and spiders are known to be very sensitive to these factors (carabid beetles: Thiele 1977; Jukes et al. 2001; spiders: Scheidler 1990; Grill et al. 2005), impacts of C. introflexus encroachment on species compositions might be assumed. Moreover, especially dry and open habitats as the studied acidic coastal dunes, harbor several specialized xerothermic carabid (Desender 1996; Müller-Motzfeld 2004; Trost 2004) and spider species (Almquist 1973; Bonte et al. 2000, 2003; Buchholz and Kreuels 2009; Buchholz 2010). Furthermore, many carabid and spider species have low space requirements resulting in small-scale habitat use (Almquist 1973; Juen and Traugott 2004; Mazia et al. 2006; Bates et al. 2007; Ziesche and Roth 2008; Muff et al. 2009). Hence, both taxa are useful indicators for small-scale shifts in habitats such as the shifts caused by moss encroachment in acidic coastal dunes.
The aim of this study was to analyze the possible impact of Campylopus introflexus on carabid beetles and spiders. Species composition was compared in (a) invaded, moss-rich sites (C. introflexus) and (b) native, lichen-rich sites using pitfall trap data. The following research questions were addressed: (a) Is diversity of carabid beetles and spiders reduced in the invaded, structure-poor sites? Do abundance patterns differ between the two acidic coastal dune habitat types? (b) Is the species composition of carabid beetles and spiders affected by the moss invasion? (c) Which species are mostly affected by the invasion? Are phytophagous carabid beetle species negatively affected by the moss invasion (which is known to hinder the germination of grasses)?
Materials and methods
Study area and plots
The study was conducted from 19 May to 28 July 2009 in an anthropo-zoogenic coastal heath (total area: approx. 200 ha) on the southern Baltic island of Hiddensee (total area: approx. 16 km²), Germany (54°32′N, 13°50′E). The average annual temperature is 7.5°C and the average precipitation is 547 mm (Reinhard 1962).
The vegetation on the acidic, nutrient-poor and sandy soils (Bauer 1972) is dominated by a mosaic of extensive dwarf-shrub stands (mainly Calluna vulgaris), acidic coastal dunes, grassy heath, shrubs and isolated trees. The acidic coastal dunes predominantly feature Corynephorus canescens, Carex arenaria, and cryptogams. In particular lichens of the genus Cladonia are very frequent (with more than ten species, Remke pers. comm.). The coastal heath was used as grazing ground until the beginning of the last century and is currently kept open thanks to several management measures such as shrub clearing, mowing, sod cutting and the reintroduction of sheep grazing (since 2004, with approx. 500 sheep).
Within the coastal heath a total of 22 acidic coastal dune plots were selected. Eleven of the plots had been invaded by Campylopus introflexus with a cover of >85% (henceforth referred to as CA) and, as reference, eleven native, lichen-rich plots with a cover of >85% of Cladonia spp. (henceforth referred to as LI) were selected (Fig. 1). The plots were almost level and had a homogenous vegetation structure (Kratochwil and Schwabe 2001). The size of each plot was >35 m² and the minimum distance among the plots amounted to >20 m.
Vegetation and microclimate
Characterization of vegetation was done once at the beginning of July in three 1 × 1 m randomly chosen squares per plot. Density and proportion of total vegetation, bare ground (sand), Campylopus introflexus, Cladonia spp., grasses (including sedges) and litter were estimated in percent. Height of grasses and the respective dominant cryptogams (either C. introflexus or Cladonia spp.) was measured at four randomly chosen spots within each square. Parameters were averaged for each plot for further analyses.
Microclimate measures were performed using data-loggers (Dallas Hygrochron Temperature/Humidity i-Button DS 1923-F5). One logger was placed 1 cm above the cryptogam vegetation in the center of each plot. A 15 × 15 cm transparent plastic roof was installed 1 cm above each logger to protect it from precipitation. Air temperature (°C) and relative air humidity (%) were logged hourly during the whole study period.
Sampling and determination of carabid beetles and spiders
Carabid beetles and spiders were sampled using pitfall traps. Pitfall trapping is a sampling method widely used with ground-dwelling arthropods, producing high catch numbers and very useful data about activity densities (Lövei and Sunderland 1996; New 1998). In each of the 22 plots three traps were set level with the soil surface, thus totalling 66 traps altogether. Traps were 6.5 cm in diameter and 7.5 cm deep. They were filled with a 80% ethylene–glycol-solution and a few drops of detergent. Within plots, traps had a minimum distance of >2 m to each other. Traps were emptied fortnightly (five emptyings in total).
Catches were sorted into taxa and stored in 70% alcohol. Identification to species level was done using Müller-Motzfeld (2006) for carabid beetles and Roberts (1987, 1996), Heimer and Nentwig (1991) and Almquist (2005) for spiders. Nomenclature follows Müller-Motzfeld (2006) (carabids) and Platnick (2009) (spiders).
Data analysis
The three traps per plot were treated as a unit. For statistical analysis the raw catch numbers of carabids and spiders were used. Classification of carabid beetles into the two feeding groups (mostly) carnivorous or (mostly) phytophagous follows Cole et al. (2002), Ribera et al. (1999a, b) and Müller-Motzfeld (2004).
Statistical analysis was carried out using R 2.10.1 (R Development Core Team 2009). For comparison of environmental parameters (vegetation, temperature, humidity) between the habitat types we used two-sample t-tests. For species richness estimation we used the bias-corrected Chao1 index (Chao 1984, 2005) with the software SPADE (Species Prediction and Diversity Estimation). Observed species richness, estimated species richness, total activity density and activity density of most frequent species (>30 individuals; Appendix) were analyzed using Poisson generalized linear models (GLM). Responses of most frequent species (>30 individuals) to environmental paramters were analyzed using GLM. To avoid multicollinearity, only parameters with correlations of r < 0.7 were included into the models (cover of grasses, proportion of bare soil, mean Temperature, cover of litter). Since overdispersion was detected we corrected standard errors using quasi-GLM models (Zuur et al. 2009). F-statistics were assessed using an Analysis of Deviance (Zuur et al. 2009). Comparisons of rank abundance-plots were conducted using the Kolmogorov–Smirnov two-sample test. D max represents the largest unsigned difference between the cumulative relative abundances of species of CA and LI. The critical value D α was calculated as D α = K α √[(n 1 + n 2)/(n 1 × n 2)], where K α = √[1/2 (−ln (α/2))] (see Magurran 2004).
Species overlap was compared using Jaccard's similarity index as C J = a/(a + b + c), where a is the number of species detected in both CA and LI, b is the number of species found in CA only and c is the number of species exclusively detected in LI. For ordination of species data we used non-metric multidimensional scaling (NMDS) (VEGAN (Oksanen et al. 2008) and MASS packages (Venables and Ripley 2010)). The Bray-Curtis distance was used as distance measure and a maximum number of 100 random starts was conducted in order to search for a stable solution. To test whether cryptogam type (Cover of Campylopus or Cladonia spp.) had a significant effect on species composition, a permutational multivariate analysis of variance using distance matrices (ADONIS) was conducted. To analyse species composition and feeding groups, we only used species with ≥3 individuals per plot. In the following always means and standard errors were shown.
Results
Vegetation and microclimate
All vegetation parameters except litter cover differed significantly between CA and LI (Table 1). Total vegetation cover reached almost 100% in both habitat types. Consequently, the proportion of bare soil was low in both habitats but slightly higher in CA. Due to the plot selection criterion (>85% cover of either Campylopus introflexus or Cladonia ssp.), the cover of cryptogams differed highly significantly between habitat types. With 16%, cover of grasses in LI was nearly twice as large as in CA; this difference was highly significant too. Finally, height of grasses in LI was significantly higher than in CA.
Microclimate in LI was drier and hotter, while it was more balanced in CA (Table 2). Mean temperature in LI was about 2°C and maximum temperature (>60°C) about 10°C higher than in CA. Mean and minimum air humidity was higher in CA, while maximum air humidity did not differ significantly between habitat types.
Carabid beetles
Capture statistics
A total of 1,846 individuals belonging to 39 species were caught. The most frequent species was Calathus erratus with 29% of the total catch followed by Syntomus foveatus (20%), Calathus fuscipes (10%), Amara tibialis (7%) and Masoreus wetterhallii (5%). Another 8 species had frequencies between 1 and 5% and made up for another 21% of the total catch. 26 species were very rare (each <1%, together 7% of total catch). The number of individuals caught per plot ranged from 21 (CA1) to 197 (LI3).
Diversity and abundance patterns
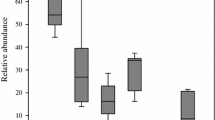
The total number of detected species was 32 in both habitats. The mean number of observed species in LI (14.9 ± 0.9) was significantly higher than in CA (10.8 ± 1.1) (GLM, F = 8.396, P = 0.009). On the other hand, the mean estimated species number in LI (Chao1: 21.1 ± 2.2) was not significantly higher than in CA (17.0 ± 2.5) (GLM, F = 1.469, P = 0.240). The same applied for the mean individual number (LI: 90.3 ± 12.1; CA: 77.5 ± 12.2; GLM, F = 0.546, P = 0.468). Comparison of shapes of the rank abundance-plots (Fig. 2a) illustrated a slightly more even species abundance pattern of carabids in LI. The shape of carabid abundance data of CA was steeper (and less even) with very high dominance of the two species Calathus erratus and Syntomus foveatus (together 63%). However, comparison of rank-abundance plots resulted in no significant differences (Kolmogorov–Smirnov two-sample test: D max = 0.2554, D α = 0.3395, P > 0.05).
Rank-abundance plots of (a) carabid beetle abundances and (b) spider abundances of acidic coastal dunes invaded by Campylopus introflexus (CA) and native, lichen-rich acidic coastal dunes (LI). Relative abundance (=activity density) of species is plotted using a log10 scale. For carabid beetles differences of shapes were not significant (Kolmogorov–Smirnov two-sample test: D max = 0.2554, D α = 0.3395, P > 0.05). Shapes of spiders differed significantly (Kolmogorov–Smirnov two-sample test: D max = 0.4163, D α = 0.3920, P < 0.001)
Species composition
Jaccard's index (0.641) showed a dissimilarity between carabid assemblages of CA and LI. NMDS ordination (stress: 10.08; 3 dimensions) based on species activity densities showed a clear and nearly complete separation of both habitat types (Fig. 3). Invaded CA could be found on the left part of the ordination plot, while the native LI were plotted on the right. Cover of cryptogam type (Campylopus, Cladonia spp.) had a significant effect on species composition (ADONIS, F = 3.985, P < 0.001, permutations = 9,999).
In CA most of the individuals found were carnivorous (90%) while the proportion was more balanced in LI (65% carnivorous and 35% phytophagous).
Species response to habitat parameters
Total abundances of analyzed phytophagous carabid beetles (total of six species) and four single phytophagous species showed significant positive responses to the cover of grasses (Fig. 4; Table 3); for Amara lunicollis a trend could be observed. For total abundances of generalist predators no significant responses could be detected. As only carnivorous species Calathus melanocephalus were positively related to the cover of grasses (trend for Calathus fuscipes) while Cicindela campestris showed a negative response. Two species (Calathus erratus, Trechus quadristriatus) were positively related to the proportion of bare soil.
In accordance to the species relationships to the environmental parameters, seven species showed significantly different distribution patterns (Appendix). Two of them, the small and carnivorous Trechus quadristriatus and Masoreus wetterhallii displayed higher numbers of individuals in CA. Three phytophagous species, Amara curta, A. lunicollis and Harpalus neglectus, were more frequent in LI, as well as Calathus fuscipes and C. melanocephalus.
Spiders
Capture statistics
Overall, 66 spider species and 2,682 individuals were caught. Pardosa monticola was the most dominant species and made up 60% of the total catch (1,590 individuals). Further frequent species were Trichopterna cito (11%), Zelotus electus (5%), Zelotus longipes (5%), Talavera aequipes (2%) and Micaria lenzi (2%). An additional 30 species were found with ≥3 individuals and comprised 14% of the catch while the remaining 30 species were found with only one or two individuals. The fewest individuals were found in one invaded plot (CA2, 24 individuals) and the most individuals (313) could be detected in a native plot (LI5).
Diversity and abundance patterns
In LI (18.5 ± 1.1) the mean number of observed spider species was significantly higher than in CA (13.6 ± 0.9) (GLM, F = 12.055, P = 0.002). Also, the mean individual number in LI (177.4 ± 22.2) was about three times higher than in CA (66.5 ± 9.7) (GLM, F = 24.947, P < 0.001). In contrast, the mean estimated species number in LI (Chao1: 27.5 ± 1.8) was not significantly higher than in CA (23.1 ± 4.3) (GLM, F = 0.857, P = 0.366). Rank abundance-plots differed significantly between CA and LI (Kolmogorov–Smirnov two-sample test: D max = 0.4163, D α = 0.3920, P < 0.001). LI-plots were clearly dominated by the most abundant species Pardosa monticola.
Species composition
Of the 66 spider species 33 were found in both habitat types (Jaccard index = 0.5). Based on the NMDS (stress: 10.67, 3 dimensions) both habitat types could be clearly separated. Invaded plots of CA were grouped on the right of the ordination plot while native plots of LI were found on the left (Fig. 5). Just as for carabid beetles, cover of cryptogam type (Campylopus, Cladonia spp.) had a highly significant effect on spider species composition (ADONIS, F = 13.19, P < 0.001, permutations = 9,999).
Species response to habitat parameters
Two frequent spider species (Dictyna latens, Pardosa monticola) were both positively correlated with the cover of grasses and negeatively with the proportion of bare soil (Table 3). A trend for a positive relation to grass cover could be observed for P. nigriceps and Talavera aequipes. In contrast, Trichopterna cito and Xysticus kochi showed a negative response to the grass cover. No spider species showed a significant response to the mean temperature or the cover of litter.
Seven spider species differed significantly in number of individuals between habitat types (Appendix). Five species had significantly higher activity densities in native plots. Among these were the Dictynidae species Dictyna latens and both Pardosa species P. monticola and P. nigriceps, which is in accordance to their significant positive relations to grass cover. On the other hand, Micaria lenzi and Trichopterna cito showed higher activity densities in CA.
Discussion
Vegetation and microclimate
Vegetation and microclimate are among the most important factors determining the occurrence patterns of carabid beetles and spiders (Almquist 1973; Thiele 1977; Grill et al. 2005; Beals 2006; De Mas et al. 2009). Although both habitat types analyzed in this study were closely related developments of acidic coastal dunes, they differed remarkably in some parameters. Obviously the main difference was the high cover of Campylopus introflexus in invaded plots as compared to the high cover of Cladonia ssp. in lichen-rich, native plots. The dense moss carpets were lower than the lichens and formed a poorly structured two-dimensional surface, which is described as a “shroud” by Pott (1995). The grass cover in invaded plots was only half as large as in native, lichen-rich plots. This is in accordance with studies in inland dunes in the Netherlands and can be explained by the negative effect of the dense moss carpets on the germination and development of higher plants (Biermann and Daniëls 2001). In contrast, many of the “volume lichens” (Hasse 2005) of the genus Cladonia are higher, highly branched and build up a three-dimensional structure (Wirth 1995). The lichen stands are often rich in species (Wirth 1995)—in the acidic coastal dunes on the island of Hiddensee a minimum of ten Cladonia spp. occur (Remke pers. comm.).
As acidic coastal dunes are open, dry and hot habitats they have extreme microclimatic conditions. Microclimate depends on the structure of the vegetation (e.g. light and air penetrability) and can differ even at a very small scale (Meissner 1998). Higher vegetation normally results in lower temperatures near the soil surface (Frey and Lösch 1998), as do bright colors (Lange 1954). Therefore, lower temperatures should be expected in the lichen-rich plots (brighter and higher), but our results showed the opposite: Native, lichen-rich plots were drier and hotter and especially the maximum temperatures (>60°C) were higher than in moss invaded plots, which showed a more balanced and moderate microclimate. An explanation might be the fact, that C. introflexus has leaves tapering in long and whitish hair-points. These hair-points reflect a big part of the insolation and the moss appears brighter. In addition, the moss is able to store water in hyalocytes and gills on the lower side of the leaf protecting it from rapid drying (Frahm 2001). Such traits are absent in lichens (Wirth 1995). As a result, air humidity was probably higher above the moss and the evaporation chill might have lead to lower temperatures and more moderate microclimate conditions (Heijmans et al. 2004).
Carabid beetles and spiders
The Northern hemisphere invader Campylopus introflexus modifies the abiotic and biotic conditions in dry, sandy and lowgrowing habitats (Hasse 2007, see above). Because many carabid and spider species are highly specialized (e.g. Bonn and Kleinwächter 1999; Bonn and Schröder 2001; Entling et al. 2007; Muff et al. 2009) they are consistently affected by the moss-encroachment.
Species richness of carabid beetles tended to be reduced in invaded, moss-rich plots as compared to native, lichen-rich plots (however, estimated species richness did not differ significantly). Furthermore, species composition differed markedly between both habitat types, which is indicated by the clear separation by the NMDS ordination and a very moderate species overlap shown by the Jaccard index. An explanation for these patterns might lie in the activity densities of phytophagous carabid species. Most (predominantly) phytophagus species of the genera Amara and Harpalus were more abundant in the native, lichen-rich sites (except H. solitaris and H. servus). Differences were significant for A. curta, A. lunicollis and H. neglectus, the latter being a regionally rare and endangered species (Müller-Motzfeld and Schmidt 2008) typical dune species (Turin 2000). Both total phytaphagous and most of the single phytophagous carabid beetle species showed significant positive relations to the cover of grasses, which was higher in native sites. Therefore, it can be assumed that the food supply for phytophagous carabids is limited by the moss encroachment. Besides the fact that grasses were less abundant in these plots (see above), it also seems possible that seeds get caught in the highly structured and branched lichens. This might also be true for Calluna seeds from the surrounding heath vegetation, which are an important food source for e.g. A. lunicollis and C. melanocephalus (occurring exclusively in native sites) (Kratochwil and Schwabe 2001). On the other hand, seeds and other possibly unfixed herbal nutrition could easily be blown away on the poorly structured surface of moss encroached sites—especially under the frequently windy conditions in such coastal regions.
Besides food supply, the vegetation structure may also influence the occurrence of certain species. Especially larger carabid beetles might be able to easily find an effective shelter from predators (e.g. birds and reptiles; Lövei and Sunderland 1996) and harsh weather conditions (heavy rain and wind) within the three-dimensional structure of the lichen vegetation.
The more extreme microclimate of the native, lichen-rich plots could also have a positive effect. In particular during spring/early summer (study period) and late summer/fall—the main activity periods of most carabid species—the soil surface in these habitats warms quickly up and therefore might produce a higher activity (Thiele 1977). In a related study by Vogels et al. (2005), carabid activity was lower in Campylopus introflexus encroached sites as compared to a reference dry grassland site. In our study, numbers of individuals did not differ between habitat types. But as pitfall catches provide data of activity densities (Lövei and Sunderland 1996; Lang 2000) they are biased towards species with high activities, which in turn might be enhanced in vegetation with low physical resistance. Since the vegetation of the native plots was more richly structured and thus exhibited a higher resistance than the moss-encroached sites, the activity densities observed in native sites might underestimate the actual activity densities as compared to the densities observed in moss-encroached sites.
Just as for the carabid beetles, observed species richness of spiders was reduced in moss-encroached sites. Activity density was higher in native sites, too. These observations may undoubtedly be ascribed to the very dominant species Pardosa monticola, which show a positive response to the grass cover. The dominance of this species also explains the differences between rank-abundance plots of both habitat types, where the native, lichen rich sites show a less even distribution. Nevertheless, the denser vegetation structure in native sites could favor higher activity densities of certain species (Bell et al. 1997), as described above for carabid beetles. For example, the wolf spider P. nigriceps can not only be seen running rapidly on the ground, but it can also be found in low vegetation (such as Calluna heath), where it is able to jump from leaf to leaf (Roberts 1996). Lichens and the higher proportion of grasses might also offer favorable structures for web-building spiders (Uetz 1991; Jiménez-Valverde and Lobo 2007) like the cribellate species of the family Dictynidae, the regionally rare Argenna subnigra and Dictyna latens (Martin 1993; Staudt 2009). This assumption is supported by the positive response of the latter species with the cover of grasses in our study. In addition, microclimatic conditions might be favorable for spiders in lichen-rich sites: most importantly, spiders there can find shelter from the wind—note that many species are sensitive to the wind and therefore try to avoid it (Almquist 1971).
In fact, species assemblages of both habitat types were clearly separated and shared only half of their species. This is significant, as both habitats are nevertheless very closely related habitat types. But these differences in species composition confirm the assumptions that micro-scale habitat relations are important for some highly specialized spider species (e.g. Finch 2008; Muff et al. 2009).
In dry habitats, such as acidic coastal dunes, species are well adapted to extreme environmental conditions, thus enabling an exclusive occurrence in these habitats (Almquist 1973; Bellmann 1997; Finch 2008). The shift in species composition caused by the moss-encroachment comes along with an almost complete displacement of some species. While species like Argenna subnigra, Dictyna latens and Talavera aequipes occurred almost exclusively in native, lichen-rich sites, the equivalent was true for Micaria lenzi and Sitticus saltator in invaded sites. Consequently, these species could be used as indicators of moss invasion.
Conclusion
In our study we could show that the invasion of Campylopus introflexus not only affects vegetation structure and composition (c.f. Hasse 2005, 2007; Ketner-Oostra and Sykora 2004, 2008) but also alters microclimate conditions. In combination, this caused a shift in species composition and reduced richness of carabid beetle and spider species. This can be explained by lower activitiy densities of phytophagous carabid species (Amara and Harpalus) and the quasi-absence of some web-building spiders species in moss-encroached sites, which could be found in native sites. In addition, activity densities of spiders (in particular of Pardosa wolf spiders) were lower in invaded sites. We conclude that, in the case of high dominance of Campylopus introflexus (covering large areas), the moss invasion might have a strong impact on typical arthropod species (Schirmel submitted) and assemblages of the formerly lichen-rich acidic coastal dunes now under EU protection. This might be especially true in coastal areas with high tourism or similar usage, where trampling or similar disturbance effects can promote the expansion of Campylopus introflexus.
References
Almquist S (1971) Resistance to desiccation in some dune-living spiders. Oikos 22:225–229
Almquist S (1973) Habitat selection by spiders on Coastal Sand Dunes in Scania, Sweden. Entomol Scand 4:134–154
Almquist S (2005) Swedish Araneae, part 1: families Atypidae to Hahniidae (Linyphiidae excluded). Insect Syst Evol Suppl 62:1–284
Bates AJ, Sadler JP, Perry JN, Fowles AP (2007) The microspatial distribution of beetles (Coleoptera) on exposed riverine sediments (ERS). Eur J Entomol 104:479–487
Bauer L (ed) (1972) Handbuch der Naturschutzgebiete der Deutschen Demokratischen Republik. Band 1: Bezirke Rostock, Schwerin und Neubrandenburg. Urania-Verlag, Leipzig
Beals ML (2006) Understanding community structure: a data-driven multivariate approach. Oecologia 150:484–495
Bell JR, Haughton AJ, Cullen WR, Wheater CP (1997) The zonation and ecology of sand-dune spider community. In: Proceedings of the 17th European colloquium of arachnology. pp 261–266
Bellmann H (1997) Zum Vorkommen dünenspezifischer Arthropoden in Mitteleuropa. Mitt Dtsch Ges Allg Angew Ent 11:839–842
Biermann R, Daniëls FJA (1997) Changes in a lichen-rich dry sand grassland vegetation with special reference to lichen synusiae and Campylopus introflexus. Phytocoenologia 27:257–273
Biermann R, Daniëls FJA (2001) Vegetationsdynamik im Spergulo-Corynephoretum unter besonderer Berücksichtigung des neophytischen Laubmooses Campylopus introflexus. Braunschweiger Geobot Arb 8:27–37
Bonn A, Kleinwächter M (1999) Microhabitat distribution of spider and ground beetle assemblages (Araneae, Carabidae) on frequently inundated river banks of the River Elbe. Z Ökologie Naturschutz 8:109–123
Bonn A, Schröder B (2001) Habitat models and their transfer for single- and multi-species groups: a case study of carabids in an alluvial forest. Ecography 24:483–496
Bonte D, Maelfait J-P, Hoffmann M (2000) Seasonal and diurnal migration patterns of the spider (Araneae) fauna of coastal grey dunes. Ekológia (Bratislava) 19(4):5–16
Bonte D, Criel P, Van Thournout I, Maelfait J-P (2003) Regional and local variation of spider assemblages (Aranae) from coastal grey dunes along the North Sea. J Biogeogr 30:901–911. doi:10.1046/j.1365-2699.2003.00885.x
Buchholz S (2010) Ground spider assemblages as indicators for habitat structure in inland sand ecosystems. Biodivers Conserv. doi:10.1007/s10531-010-9860-7
Buchholz S, Kreuels M (2009) Diversity and distribution of spiders (Arachnida: Araneae) in dry ecosystems of North Rhine-Westphalia. Arachnol Mitt 38:8–28
Buchholz H, Hannig K, Schirmel J (2009) Ground beetle assemblages of peat bog remnants in Northwest Germany (Coleoptera: Carabidae). Entomol Generalis 32(2):127–144
Bultmann TL, DeWitt DJ (2008) Effect of an invasive ground cover plant on the abundance and diversity of a forest floor spider assemblage. Biol Invasions 10:749–756. doi:10.1007/s10530-007-9168-z
Chao A (1984) Nonparametric estimation of the number of classes in a population. Scand J Stat 11:265–270
Chao A (2005) Species estimation and applications. In: Balakrishnan N, Read CB, Vidakovic B (eds) Encyclopedia of statistical sciences, vol 12, 2nd edn. Wiley, New York, pp 7907–7916
Cole LJ, McCracken DI, Dennis P, Downie IS, Griffin AL, Foster GN, Murphy KJ, Waterhouse T (2002) Relationships between agricultural management and ecological groups of ground beetles (Coleoptera: Carabidae) on Scottish farmland. Agric Ecosyst Environ 93:323–336. doi:10.1016/S0167-8809(01)00333-4
De Mas E, Chust G, Pretus JL, Ribera C (2009) Spatial modelling of spider biodiversity: matters of scale. Biodivers Conserv 18:1945–1962
De Vries W, Klijn JA, Kros H (1994) Simulation of the long-term impact of atmospheric deposition on dune ecosystems in the Netherlands. J Appl Ecol 31:59–73
Desender KRC (1996) Diversity and Dynamics of coastal dune carabids. Ann Zool Fennici 33:65–75
Ellenberg H (1996) Vegetation Mitteleuropas mit den Alpen in ökologischer, dynamischer und historischer Sicht. Ulmer, Stuttgart
Entling W, Schmidt MH, Bacher S, Brandl R, Nentwig W (2007) Niche properties of Central European spiders: shading, moisture and the evolution of the habitat niche. Global Ecol Biogeogr 16:440–448
Equihua M, Usher MB (1993) Impact of carpets of the invasive moss Campylopus introflexus on Calluna vulgaris regeneration. J Ecol 81:359–365
Finch OD (2008) Webspinnen, Weberknechte und Pseudoskorpione der Ostfriesischen Inseln (Arachnida: Araneae, Opilionida, Pseudoscorpionida). Schrift R Nationalpark Niedersächs Wattenmeer 11:103–112
Frahm JP (1980) Synopsis of the genus Campylopus in North America north of Mexico. Bryologist 83:570–588
Frahm JP (2001) Biologie der Moose. Spektrum, Berlin
Frey W, Lösch R (1998) Lehrbuch der Geobotanik: Pflanze und Vegetation in Raum und Zeit. Gustav Fischer, Stuttgart
Grill A, Knoflach B, Cleary DFR, Kati V (2005) Butterfly, spider, and plant communities in different land-use types in Sardinia, Italy. Biodivers Conserv 14:1281–1300
Gu W, Sang W, Liang H, Axmacher JC (2008) Effects of Crofton weed Ageratina adenophora on assemblages of Carabidae (Coleoptera) in the Yunnan Province, South China. Agric Ecosyst Environ 124:173–178. doi:10.1016/j.agee.2007.09.004
Hansen AK, Ortega YK, Six DL (2009) Comparison of Ground Beetle (Coleoptera: Carabidae) Assemblages in Rocky Mountain Savannas Invaded and Un-invaded by an Exotic Forb, Spotted Knapweed. Northwest Sci 83(4):348–360. doi:10.3955/046.083.0406
Hasse T (2005) Charakterisierung der Sukzessionsstadien im Spergulo-Corynephoretum (Silbergrasfluren) unter besonderer Berücksichtigung der Flechten. Tuexenia 25:407–424
Hasse T (2007) Campylopus introflexus invasion in a dune grassland: Succession, disturbance and relevance of existing plant invader concepts. Herzogia 20:305–315
Hassel K, Söderström L (2005) The expansion of the alien mosses Orthodontium lineare and Campylopus introflexus in Britain and continental Europe. J Hattori Bot Lab 97:183–193
Heijmans MMPD, Arp WJ, Chapin FS (2004) Controls on moss evaporation in a boreal black spruce forest. Glob Biogeochem Cycles 18:1524–1530
Heimer S, Nentwig W (1991) Spinnen Mitteleuropas: ein Bestimmungsbuch. Parey, Berlin
Horvath R, Magura T, Szinetar C, Tothmeresz B (2009) Spiders are not less diverse in small and isolated grasslands, but less diverse in overgrazed grasslands: a field study (East Hungary, Nyirseg). Agric Ecosyst Environ 130:16–22. doi:10.1016/j.agee.2008.11.011
Hsieh Y-L, Lin Y-S, Tso I-M (2003) Ground spider diversity in the Kenting uplifted coral reef forest, Taiwan: a comparison between habitats receiving various disturbances. Biodivers Conserv 12:2173–2194. doi:10.1023/A:1024591311548
Huber C, Schulze C, Baumgarten M (2007) The effect of femel- and small scale clear-cutting on ground dwelling spider communities in a Norway spruce forest in Southern Germany. Biodivers Conserv 16:3653–3680
Irmler U, Gürlich S (2004) Die ökologische Einordnung der Laufkäfer (Coleoptera: Carabidae) in Schleswig-Holstein. Faunist-Ökol Mitt 32:1–117
Jiménez-Valverde A, Lobo JM (2007) Determinants of local spider (Araneidae and Thomisidae) species richness on a regional scale: climate and altitude vs. habitat structure. Ecol Ent 32:113–122
Juen A, Traugott M (2004) Spatial distribution of epigaeic predators in a small field in relation to season and surrounding crops. Agric Ecosyst Environ 103:613–620
Jukes MR, Peace AJ, Ferris R (2001) Carabid beetle communities associated with coniferous plantations in Britain: the influence of site, ground vegetation and stand structure. For Ecol Manage 148:271–286
Ketner-Oostra R, Sykora KV (2004) Decline of lichen-diversity in calcium-poor coastal dune vegetation since the 1970s, related to grass and moss encroachment. Phytocoenologia 34:521–549. doi:10.1127/0340-269X/2004/0034-0521
Ketner-Oostra R, Sykora KV (2008) Vegetation change in a lichen-rich inland drift sand area in the Netherlands. Phytocoenologia 38:267–286. doi:10.1127/0340-269X/2008/0038-0267
Kratochwil A, Schwabe A (2001) Ökologie der Lebensgemeinschaften. Ulmer, Stuttgart
Lang A (2000) The pitfalls of pitfalls: a comparison of pitfall trap catches and absolute density estimates of epigeal invertebrate predators in arable land. J Pest Sci 73:99–106
Lange OL (1954) Einige Messungen zum Wärmehaushalt poikilohydrer Flechten und Moose. Arch Meteorol Geophys Bioklimatol Serie B 5(2):182–190
Lövei GL, Sunderland KD (1996) Ecology and behavior of ground beetles (Coleoptera: Carabidae). Ann Rev Entomol 41:231–256. doi:10.1146/annurev.en.41.010196.001311
Maes D, Bonte D (2006) Using distribution patterns of five threatened invertebrates in a highly fragmented dune landscape to develop a multispecies conservation approach. Biol Conserv 133:490–499. doi:10.1016/j.biocon.2006.08.001
Maes D, Ghesquiere A, Logie M, Bonte D (2006) Habitat use and mobility of two threatened coastal dune insects: implications for conservation. J Insect Conserv 10:105–115. doi:10.1007/s10841-006-6287-2
Magurran AE (2004) Measuring biological diversity. Blackwell, Oxford
Martin D (1993) Rote Liste der gefährdeten Spinnen (Araneae) Mecklenburg-Vorpommerns. In: Mecklenburg-Vorpommern U (ed) Rote Liste der gefährdeten Spinnen (Araneae) Mecklenburg-Vorpommerns. pp 1–41
Mazia CN, Chaneton EJ, Kitzberger T (2006) Small-scale habitat use and assemblage structure of ground-dwelling beetles in a Patagonian shrub steppe. J Arid Environ 67:177–194. doi:10.1016/j.jaridenv.2006.02.006
Meissner A (1998) Die Bedeutung der Raumstruktur für die Habitatwahl von Lauf und Kurzflügelkäfern (Coleoptera: Carabidae, Staphylinidae). Freilandökologische und experimentelle Untersuchung einer Niedermoorzönose. Dissertation, University Berlin
Mgobozi MP, Somers MJ, Dippenaar-Schoeman AS (2008) Spider responses to alien plant invasion: the effect of short- and long-term Chromolaena odorata invasion and management. J Appl Ecol 45:1189–1197. doi:10.1111/j.1365-2664.2008.01486.x
Mossakowski D, Främbs H (1993) Carabiden als Indikatoren der Auswirkungen von Wiedervernässerungsmaßnahmen auf die Fauna im Leegmoor. Nat Schutz Landsch Pfl Nieders 29:79–114
Muff P, Kropf C, Frick H, Nentwig W, Schmidt-Entling MH (2009) Co-existence of divergent communities at natural boundaries: spider (Arachnida: Araneae) diversity across an alpine timberline. Insect Conserv Diver 2:36–44
Muller S, Dutoit T, Alard D, Grévilliot F (1998) Restoration and rehabilitation of species-rich grassland ecosystems in France: a review. Restor Ecol 6:94–101. doi:10.1046/j.1526-100x.1998.06112.x
Müller-Motzfeld G (2004) Xerotherme Laufkäfer in Deutschland—Verbreitung und Gefährdung. Angew Carabidol 3:27–44
Müller-Motzfeld G (2006) Band 2, Adephaga 1: Carabidae (Laufkäfer). In: Freude H, Harde KW, Lohse GA, Klausnitzer B (eds) Die Käfer Mitteleuropas. Spektrum-Verlag, Heidelberg
Müller-Motzfeld G, Schmidt J (2008) Rote Liste der Laufkäfer Mecklenburg-Vorpommerns. Turo Print GmbH, Schwerin
Negro M, Isaia M, Palestrini C, Rolando A (2009) The impact of forest ski-pistes on diversity of ground-dwelling arthropods and small mammals in the Alps. Biodivers Conserv. doi:10.1007/s10531-009-9608-4
New TR (1998) Invertebrate surveys for conservation. Oxford University Press, Oxford
Oksanen J, Kindt R, Legendre P, O’Hara B, Simpson GL, Solymos P, Stevens MH, Wagner H (2008) The vegan Package Version 1.15-0. Online at: http://cran.r-project.org/, http://vegan.r-forge.r-project.org/ (15/02/2010)
Petillon J, Ysnel F, Canard A, Lefeuvre J-C (2005) Impact of an invasive plant (Elymus athericus) on the conservation value of tidal salt marshes in western France and implications for management: response of spider populations. Biol Conserv 126:103–117. doi:10.1016/j.biocon.2005.05.003
Platnick NI (2009) The world spider catalog, Version 10.0. American Museum of Natural History. Online at: http://research.amnh.org/entomology/spiders/catalog/index.html (05/11/2009)
Pott R (1995) Die Pflanzengesellschaften Deutschlands. Ulmer, Stuttgart
R Development Core Team (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Reinhard H (1962) Klimatologie. Atlas der Bezirke Rostock, Schwerin und Neubrandenburg. VEB Topographischer Dienst, Schwerin
Remke E, Brouwer E, Kooijman A, Blindow I, Esselink H, Roelofs JGM (2009) Even low to medium nitrogen deposition impacts vegetation of dry, coastal dunes around the Baltic Sea. Environ Pollut 157:792–800. doi:10.1016/j.envpol.2008.11.020
Ribera I, Foster GN, Downie IS, McCracken DI, Abernethy VJ (1999a) A comparative study of the morphology and life traits of Scottish ground beetles (Coleoptera, Carabidae). Annal Zool Fennici 36:21–37
Ribera I, McCracken DI, Foster GN, Downie IS, Abernethy VJ (1999b) Morphological diversity of ground beetles (Coleoptera: Carabidae) in Scottish agricultural land. J Zoology 247:1–18. doi:10.1017/S0952836999001016
Richards PW (1963) Campylopus introflexus (Hedw.) and C. polytrichoides De Not. in the British Islands: a preliminary account. Trans Brit Bryol Soc 3:404–417
Riksen M, Ketner-Oostra R, van Turnhout C, Nijssen M, Goossens D, Jungerius PD, Spaan W (2006) Will we lose the last active inland drift sands of Western Europe? The origin and development of the inland drift-sand ecotype in the Netherlands. Landsc Ecol 21:431–447. doi:10.1007/s10980-005-2895-6
Roberts MJ (1987) The spiders of Great Britain and Ireland. Volume 2: Linyphiidae and checklist. Harley Books, Colchester
Roberts MJ (1996) Spiders of Britain and Northern Europe. Collins, London
Scheidler M (1990) Influence of habitat structure and vegetation architecture on spiders. Zool Anz 225:333–340
Ssymank A, Hauke U, Rückriem C, Schröder E (1998) Das europäische Schutzgebietssystem NATURA 2000. Schrift R Landsch Pfl Nat Schutz 53:1–560
Staudt A (2009) Nachweiskarten der Spinnentiere Deutschlands. Online at http://www.spiderling.de/arages
Thiele HU (1977) Carabid beetles in their environments. A study on habitat selection by adaptations in physiology and behaviour. Springer, Heidelberg
Topp W, Kappes H, Rogers F (2008) Response of ground-dwelling beetle (Coleoptera) assemblages to giant knotweed (Reynouria spp.) invasion. Biol Invasions 10:381–390. doi:10.1007/s10530-007-9137-6
Trost M (2004) Differenzierung der Carabidenfauna mitteldeutscher Xerothermhabitate mit besonderer Berücksichtigung Sachsen-Anhalts. Angew Carabidol 3:95–114
Turin H (2000) De Nederlandse Loopkevers. Verspreiding en oecologie (Coleoptera: Carabidae). European Invertebrate Survey Nederland, Leiden/Nederland
Uetz GW (1991) Habitat structure and spider foraging. In: Bell SS, McCoy ED, Mushinsky HR (eds) Habitat structure: the physical arrangement of objects in space. Chapman & Hall, New York, pp 325–348
Van der Meulen F, van der Hagen H, Kruijsen B (1987) Campylopus introflexu. Invasion of a moss in Dutch coastal dunes. Proc Kon Ned Acad Wetensch 90:73–80
Venables WN, Ripley BD (2010) Package ‘Mass’. Online at: http://cran.r-project.org/web/packages/MASS/MASS.pdf
Vogels J, Nijssen M, Verberk W, Esselink H (2005) Effects of moss encroachment by Campylopus introflexus on soil-entomofauna of dry-dune grasslands (Violo-corynephoretum). Proc Neth Entomol Soc Meet 16:71–80
Warui CM, Villet MR, Young TP, Jocque R (2005) Influence of grazing by large mammals on the spider community of a Kenyan savanna biome. J Arachnol 33:269–279
Wirth V (1995) Die Flechten Baden-Württembergs, Teil 1. Ulmer, Stuttgart
Schirmel J (submitted) Response of the grasshopper Myrmeleotettix maculatus (Orthoptera: Acrididae) to invasion by the exotic moss Campylopus introflexus in acidic coastal dunes
Ziesche TM, Roth M (2008) Influence of environmental parameters on smallscale distribution of soil-dwelling spiders in forests: what makes the difference, tree species or microhabitat? For Ecol Manage 255:738–752. doi:10.1016/j.foreco.2007.09.060
Zuur AF, Ieno IN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, Berlin
Acknowledgments
The authors would like to thank J. Schalajda, R. Baumgartner and two anonymous reviewers for helpful comments on an earlier version of the manuscript. The study was conducted as part of the research project “Biodiversity, Ecology and Management of Coastal Habitats of the Baltic Sea” and financially supported by the Bauer-Hollmann-Foundation. We are grateful to the national park “Vorpommersche Boddenlandschaft” for the permission to conduct the study in the protected area.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 4.
Rights and permissions
About this article
Cite this article
Schirmel, J., Timler, L. & Buchholz, S. Impact of the invasive moss Campylopus introflexus on carabid beetles (Coleoptera: Carabidae) and spiders (Araneae) in acidic coastal dunes at the southern Baltic Sea. Biol Invasions 13, 605–620 (2011). https://doi.org/10.1007/s10530-010-9852-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-010-9852-2