Abstract
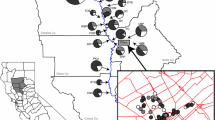
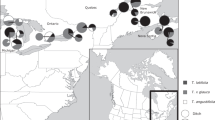
Hybridization, both within and between taxa, can be an important evolutionary stimulus for bioinvasions. Novel intra-taxon hybridizations may arise either between formerly allopatric introduced lineages, or between native and introduced lineages. The latter can occur following a cryptic invasion of a non-native lineage, such as the nineteenth century introduction to North America of a European lineage of the common reed Phragmites australis. Previous studies found no evidence of natural hybridization between native and introduced lineages of P. australis, but produced some F1 hybrids under experimental conditions when the seed parent was native and the pollen parent was introduced. In this study we used microsatellite data to compare genotypes of P. australis along a transect of approximately 2,000 km in eastern North America. Although hybridization appears uncommon, simulations and principle component analysis of genetic data provided strong evidence for natural hybridization at two sites adjacent to Lake Erie in which native and introduced lineages were sympatric. The seed parent was the native lineage in some cases, and the introduced lineage in other cases. There is now the potential for P. australis hybrids to become increasingly invasive, and managers should consider as a priority the removal of introduced stands from sites where they co-exist with native stands.



Similar content being viewed by others
References
Blumenthal D, Mitchell CE, Pysek P, Jarosik V (2009) Synergy between pathogen release and resource availability in plant invasion. PNAS 106:7899–7904
Clevering OA, Lissner J (1999) Taxonomy, chromosome numbers, clonal diversity and population dynamics of Phragmites australis. Aquat Bot 64:185–208
Culley TM, Hardiman NA (2009) The role of intraspecific hybridization in the evolution of invasiveness: a case study of the ornamental pear tree Pyrus calleryana. Biol Invasions 11:1107–1119
Dawson W, Burslem D, Hulme PE (2009) Factors explaining alien plant invasion success in a tropical ecosystem differ at each stage of invasion. J Ecol 97:657–665
Dray S, Dufour AB (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22:1–20
Ellstrand NC, Schierenbeck KA (2006) Hybridization as a stimulus for the evolution of invasiveness in plants? Euphytica 148:35–46
Engloner AI (2009) Structure, growth dynamics and biomass of reed (Phragmites australis)—a review. Flora 204:331–346
Freeland JR (2005) Molecular ecology. Wiley, Chichester
Garroway CJ, Bowman J, Cascaden TJ, Holloway GL, Mahan CG, Malcolm JR, Steele MA, Turner G, Wilson PJ (2010) Climate change induced hybridization in flying squirrels. Glob Chang Biol 16:113–121
Hansen DL, Lambertini C, Jampeetong A, Brix H (2007) Clone-specific differences in Phragmites australis: effects of ploidy level and geographic origin. Aquat Bot 86:269–279
Howard RJ, Travis SE, Sikes BA (2008) Rapid growth of a Eurasian haplotype of Phragmites australis in a restored brackish marsh in Louisiana, USA. Biol Invasions 10:369–379
Ishii J, Kadono Y (2002) Factors influencing seed production of Phragmites australis. Aquat Bot 72:129–141
Jodoin Y, Lavoie C, Villeneuve P, Theriault M, Beaulieu J, Belzile F (2008) Highways as corridors and habitats for the invasive common reed Phragmites australis in Quebec, Canada. J Appl Ecol 45:459–466
Jombart T (2008) Adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24:1403–1405
Jombart T, Pontier D, Dufour AB (2009) Genetic markers in the playground of multivariate analysis. Heredity 102:330–341
Kuehn MM, Minor JE, White BN (1999) An examination of hybridization between the cattail species Typha latifolia and Typha angustifolia using random amplified polymorphic DNA and chloroplast DNA markers. Mol Ecol 8:1981–1990
Lavergne S, Molofsky J (2007) Increased genetic variation and evolutionary potential drive the success of an invasive grass. PNAS 104:3883–3888
MacDougall AS, Gilbert B, Levine JM (2009) Plant invasions and the niche. J Ecol 97:609–615
Mal TK, Narine L (2004) The biology of Canadian weeds. 129. Phragmites australis (Cav.) Trin. ex Steud. Can J Plant Sci 84:365–396
McKee J, Richards AJ (1996) Variation in seed production and germinability in common reed (Phragmites australis) in Britain and France with respect to climate. New Phytol 133:233–243
Meyerson LA, Viola DV, Brown RN (2009) Hybridization of invasive Phragmites australis with a native subspecies in North America. Biol Invasions (on-line)
Olson A, Paul J, Freeland JR (2009) Habitat preferences of cattail species and hybrids (Typha spp.) in eastern Canada. Aquat Bot 91:67–70
Patterson N, Price AL, Reich D (2006) Population structure and eigenanalysis. Plos Genet 2:2074–2093
Prentis PJ, White EM, Radford IJ, Lowe AJ, Clarke AR (2007) Can hybridisation cause local extinction: a case for demographic swamping of the Australian native Senecio pinntifolius by the invasive Senecio madagascariensis? New Phytol 176:902–912
Prentis PJ, Wilson JRU, Dormontt EE, Richardson DM, Lowe AJ (2008) Adaptive evolution in invasive species. Trends Plant Sci 13:288–294
Raicu P, Staicu S, Stoian V, Roman T (1972) The Phragmites communis Trin. chromosome complement in the Danube delta. Hydrobiol 39:83–89
Rosenthal DM, Schwarzbach AE, Donovan LA, Raymond O, Rieseberg LH (2002) Phenotypic differentiation between three ancient hybrid taxa and their parental species. Int J Plant Sci 163:387–398
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. PNAS 99:2445–2449
Saltonstall K (2003a) Genetic variation among north American Populations of Phragmites australis: implications for management. Estuaries 26:444–451
Saltonstall K (2003b) Microsatellite variation within and among North American lineages of Phragmites australis. Mol Ecol 12:1689–1702
Saltonstall K (2003c) A rapid method for identifying the origin of North American Phragmites populations using RFLP analysis. Wetlands 23:1043–1047
Schierenbeck KA, Ellstrand NC (2009) Hybridization and the evolution of invasiveness in plants and other organisms. Biol Invasions 11:1093–1105
T’Ulbure MG, Johnston CA, Auger DL (2007) Rapid invasion of a Great Lakes coastal wetland by non-native Phragmites australis and Typha. J Great Lakes Res 33:269–279
R Development Core Team (2008) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Verhoeven KJF, Biere A, Harvey JA, van der Putten WH (2009) Plant invaders and their novel natural enemies: who is naive? Ecol Lett 12:107–117
Whitney KD, Randell RA, Rieseberg LH (2006) Adaptive introgression of herbivore resistance traits in the weedy sunflower Helianthus annuus. Am Nat 167:794–807
Whyte RS, Trexel-Kroll D, Klarer DM, Shields R, Francko DA (2008) The invasion and spread of Phragmites australis during a period of low water in a lake Erie coastal wetland. J Coast Res (Special issue 55):111–120
Williams DA, Overholt WA, Cuda JP, Hughes CR (2005) Chloroplast and microsatellite DNA diversities reveal the introduction history of Brazilian peppertree (Schinus terebinthifolius) in Florida. Mol Ecol 14:3643–3656
Wilson JRU, Dormontt EE, Prentis PJ, Lowe AJ, Richardson DM (2009) Something in the way you move: dispersal pathways affect invasion success. TREE 24:136–144
Acknowledgments
Field expertise and permits were provided by Tammy Dobbie and Leonardo Cabrera Garcia from Point Pelee National Park of Canada; Rachel Carson Wildlife Refuge; Rondeau Bay Provincial Park; Paul Ashley, Canadian Wildlife Service, Big Creek National Wildlife Area. Financial support for this project was provided by NSERC and Trent University. Two anonymous reviewers provided valuable comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paul, J., Vachon, N., Garroway, C.J. et al. Molecular data provide strong evidence of natural hybridization between native and introduced lineages of Phragmites australis in North America. Biol Invasions 12, 2967–2973 (2010). https://doi.org/10.1007/s10530-010-9699-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-010-9699-6