Abstract
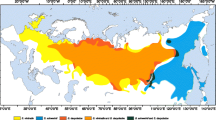
Saltcedars (Tamarix ramosissima and T. chinensis) are native to Asia, but since introduction into the USA have become common and invasive in many western riparian habitats. Recent molecular analysis of a single locus nuclear DNA sequence marker has shown that in their native range the two species are genetically distinct, but within the USA populations many of the plants (23%) are novel hybrids. Here, we used multilocus DNA markers (amplified fragment length polymorphisms) to determine the levels of introgression in USA plants. Species-specific diagnostic markers, principal coordinates analysis, and a Bayesian model-based clustering analysis all indicate a much higher incidence of hybridization (83–87%) than was revealed by the single locus marker, with USA plants forming a genetic continuum between the two parental types. Additionally, the level of introgression toward Tamarix ramosissima or T. chinensis was strongly correlated with latitude. Concordance of level of introgression was highest between principal coordinates analysis and the Bayesian analysis. The high percentage of novel hybrids may have implications for classical biological control efforts.




Similar content being viewed by others
Abbreviations
- AFLP:
-
Amplified fragment length polymorphism
- PCOA:
-
Principal coordinate analysis
- UPGMA:
-
Unweighted pair group matching algorithm
- PPCL:
-
Phosphoenolpyruvate carboxylase
References
Abbott RJ (1992) Plant invasions, interspecific hybridization and the evolution of new plant taxa. Trends Ecol Evol 7:401–405
Abbott RJ, James JK, Milne RI et al (2003) Plant introductions, hybridization and gene flow. Philos T R Soc B 358:1123–1132
Ainouche ML, Fortune PM, Salmon A, Parisod C, Grandbastien M-A, Fukunaga K, Ricou M, Misset M-T (2008) Hybridization, polyploidy and invasion: lessons from Spartina (Poaceae). Biol Invasions. doi:10.1007/s10530-008-9383-2
Allred K (2002) Identification and taxonomy of Tamarix (Tamaricaceae) in New Mexico. Desert Plants 18:26–32
Anderson EC (1949) Introgressive hybridization. Wiley, New York
Anderson EC, Thompson EA (2002) A model-based method for identifying species hybrids using multilocus genetic data. Genetics 160:1217–1229
Bailey JK, Schweitzer JA, Whitham TG (2001) Salt cedar negatively affects biodiversity of aquatic macroinvertebrates. Wetlands 21:223–331
Baum BR (1967) Introduced and naturalized tamarisks in the United States and Canada. Baileya 15:19–25
Baum BR (1978) The genus Tamarix. Israel Academy of Sciences and Humanities, Jerusalem, 209 pp
Benham JJ (2001) Genographer v. 1.6.0. http://hordeum.oscs.montana.edu/genographer/. Cited 15 Dec 2006
Bleeker W (2003) Hybridization and Rorippa austriaca (Brassicaceae) invasion in Germany. Mol Ecol 12:1831–1841
Bonin A, Bellemain E, Bronken Eidesen P et al (2004) How to track and assess genotyping errors in population genetics studies. Mol Ecol 13:3261–3273
Bonin A, Erich D, Manel S (2007) Statistical analysis of amplified fragment length polymorphism data: a toolbox for molecular ecologists and evolutionists. Mol Ecol 16:3737–3758
Bruckart W, Cavin C, Vajna L et al (2004) Differential susceptibility of Russian thistle accessions to Colletotrichum gloeosporioides. Biol Control 30:306–311
Burdon JJ, Groves RH, Cullen JM (1981) The impact of biological-control on the distribution and abundance of Chondrilla juncea in southeastern Australia. J Appl Ecol 18:957–966
Burdon JJ, Groves RH, Kaye PE et al (1984) Competition in mixtures of susceptible and resistant genotypes of Chondrilla juncea differentially infected with rust. Oecologia 64:199–203
Choler P, Erschbamer B, Tribsch A et al (2004) Genetic introgression as a potential to widen a species’ niche: insights from alpine Carex curvula. Proc Natl Acad Sci USA 101:171–176
Crins WJ (1989) The Tamaricaceae of the southeastern United States. J Arnold Arbor 70:403–425
DeLoach CJ, Carruthers RI, Dudley TL (2004) First results for control of saltcedar (Tamarix spp.) in the open field in the western United States. In: Cullen JM, Briese DT, Kriticos DJ (eds) Proceedings of the XI international symposium on biological control of weeds. CSIRO Entomology, Canberra, pp 505–513
Di Tomaso JM (1998) Impact, biology, and ecology of salt-cedar (Tamarix spp.) in the southwestern United States. Weed Technol 12:326–336
Dice L (1945) Measures of the amount of ecologic association between species. Ecology 26:297–302
Ellstrand NC, Schierenbeck KA (2000) Hybridization as a stimulus for the evolution of invasiveness in plants? Proc Natl Acad Sci USA 97:7043–7050
Gaskin JF, Schaal BA (2002) Hybrid Tamarix widespread in US invasion and undetected in native Asian range. Proc Natl Acad Sci USA 99:11256–11259
Gaskin JF, Shafroth PB (2005) Hybridization of Tamarix ramosissima and T. chinensis (saltcedars) with T. aphylla (athel) (family Tamaricaceae) in the southwestern USA determined from DNA sequence data. Madroño 52:1–10
Gobert V, Moja S, Colson M et al (2002) Hybridization in the section Mentha (Lamiaceae) inferred from AFLP markers. Am J Bot 89:2017–2023
Goolsby JA, De Barro PJ, Makinson JR et al (2006) Matching the origin of an invasive weed for selection of a herbivore haplotype for a biological control programme. Mol Ecol 15:287–297
Guo Y-P, Saukel J, Mittermayr R et al (2005) AFLP analyses demonstrate genetic divergence, hybridization, and multiple polyploidization in the evolution of Achillea (Asteraceae-Anthemideae). New Phytol 166:273–290
Hansen LB, Siegismund HR, Jorgensen RB (2003) Progressive introgression between Brassica napus (oilseed rape) and B. rapa. Heredity 91:276–283
Hillis DM, Mable BK, Larson A et al (1996) Nucleic acids IV:sequencing and cloning. In: Hillis DM, Moritz C, Mable BK (eds) Molecular systematics. Sinauer Associates, Sunderland, pp 321–381
Jensen JL, Bohonak AJ, Kelley ST (2005) Isolation by distance, web service. BMC Genet 6:13
Lee C (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391
Lewontin RC, Birch LC (1966) Hybridization as a source of variation for the adaptation to new environments. Evolution 20:315–336
Milne RI, Abbott RJ (2000) Origin and evolution of invasive naturalized material of Rhododendron ponticum L. in the British Isles. Mol Ecol 9:541–556
Moody ML, Les DH (2002) Evidence of hybridity in invasive watermilfoil (Myriophyllum) populations. Proc Natl Acad Sci USA 99:14867–14871
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. P Natl Acad Sci USA 76:5269–5273
O’Hanlon PC, Peakall R, Briese DT (1999) Amplified fragment length polymorphism (AFLP) reveals introgression in weedy Onopordum thistles: hybridization and invasion. Mol Ecol 8:1239–1246
Papa R, Troggio M, Ajmone-Marsan P et al (2005) An improved protocol for the production of AFLP markers in complex genomes by means of capillary electrophoresis. J Anim Breed Genet 122:62–68
Rieseberg LH, Kim MJ, Seiler GJ (1999) Introgression between the cultivated sunflower and a sympatric wild relative, Helianthus petiolaris (Asteraceae). Int J Plant Sci 160:102–108
Rieseberg LH, Kim SC, Randell RA et al (2007) Hybridization and the colonization of novel habitats by annual sunflowers. Genetica 129:149–165
Robinson TW (1965) Introduction, spread, and aerial extent of saltcedar (Tamarix) in the western states. US Geological Survey. Report 491-A
Roderick GK, Navajas M (2003) Genes in new environments: genetics and evolution in biological control. Nat Rev Genet 4:889–899
Rohlf FJ (1992) NTSYS-pc. Numerical taxonomy and multivariate analysis system. Version 2.11f. Exeter Software, Setauket
Sakai AK, Allendorf FW, Holt JS et al (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
SAS Institute Inc. (2000) SAS OnlineDoc, Version 8. SAS Institute, Inc., Cary
Schierenbeck K, Ellstrand NC (2008) Hybridization and the evolution of invasiveness in plants and other organisms. Biol Invasions. doi:10.1007/s10530-008-9388-x
Scotti I, Mariani A, Verona V et al (2002) AFLP markers and cytotaxonomic analysis reveal hybridisation in the genus Schoenus (Cyperaceae). Genome 45:222–228
Seehausen O (2004) Hybridization and adaptive radiation. Trends Ecol Evol 19:198–207
Shafroth PB, Cleverly J, Dudley TL et al (2005) Saltcedar removal water salvage and wildlife habitat restoration along rivers in the southwestern US. Environ Manag 35:231–246
Sloop CM, Ayres DR, Strong DR (2008) The rapid evolution of self-fertility in Spartina hybrids (Spartina alterniflora × foliosa) invading San Francisco Bay, CA. Biol Invasions. doi:10.1007/s10530-008-9385-0
Stebbins GLJ (1959) The role of hybridization in evolution. Proc Am Philos Soc 103:231–251
Templeton A (1981) Mechanisms of speciation—a population genetic approach. Annu Rev Ecol Syst 12:23–48
Tranel PJ, Wassom JJ (2001) Genetic relationships of common cocklebur accessions from the United States. Weed Sci 49:318–325
Vila M, Weber E, D’Antonio CM (2000) Conservation implications of invasion by plant hybridization. Biol Invasions 2:207–217
Vos P, Hogers R, Bleeker M et al (1995) AFLP—a new technique for DNA-fingerprinting. Nucleic Acids Res 23:4407–4414
Wang B, Porter AH (2004) An AFLP-based interspecific linkage map of sympatric, hybridizing Colias butterflies. Genetics 168:215–225
Whitney KD, Randell RA, Rieseberg LH (2006) Adaptive introgression of herbivore resistance traits in the weedy sunflower Helianthus annuus. Am Nat 167:794–807
Williams DA, Overholt WA, Cuda JP et al (2005) Chloroplast and microsatellite DNA diversities reveal the introduction history of Brazilian peppertree (Schinus terebinthifolius) in Florida. Mol Ecol 14:643–3656
Young WP, Ostberg CO, Keim P et al (2001) Genetic characterization of hybridization and introgression between anadromous rainbow trout (Oncorhynchus mykiss irideus) and coastal cutthroat trout (O. clarki clarki). Mol Ecol 10:921–930
Zavaleta E (2000) Valuing ecosystem services lost to Tamarix invasion in the United States. In: Mooney HA, Hobbs RJ (eds) Invasive species in a changing world. Island, Washington, pp 261–300
Acknowledgments
We thank D. Branson and three anonymous reviewers for helpful comments on this manuscript, Adam Birken, David Cooper, George Yatskievych, and others for donating plant samples, and Kim Mann and Jeannie Lassey for AFLP and DNA sequencing in the laboratory. This research was made possible through funding from the National Geographic Society Committee for Research and Exploration Grant # 6663-99, the Bureau of Land Management (Montana, South and North Dakota), the Bureau of Indian Affairs (Rocky Mountain Office), and U.S. Department of Agriculture National Research Initiative Competitive Grants Program, Cooperative State Research, Education, and Extension Service Grant 2000-00836.
Author information
Authors and Affiliations
Corresponding author
Additional information
John F. Gaskin and David J. Kazmer contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Gaskin, J.F., Kazmer, D.J. Introgression between invasive saltcedars (Tamarix chinensis and T. ramosissima) in the USA. Biol Invasions 11, 1121–1130 (2009). https://doi.org/10.1007/s10530-008-9384-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-008-9384-1