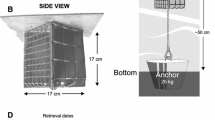
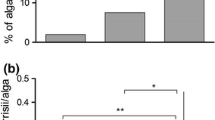
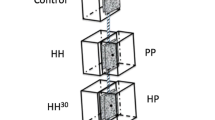
Abstract
Over the last decade, the non-native, filter-feeding crab Petrolisthes armatus invaded oyster reefs of the South Atlantic Bight at densities of thousands m−2. Mesocosm and field experiments demonstrated that P. armatus at ∼10–75% of mean summer densities: (1) suppressed growth of small oysters, biomass of benthic microalgae, and recruitment of native mud crabs, (2) enhanced oyster, mussel, and total bivalve recruitment, macroalgal cover, and survivorship of predatory oyster drills, but (3) did not affect native taxonomic richness. Laboratory feeding assays, field tethering experiments, and population changes in field and mesocosm experiments suggest that P. armatus is a preferred prey for native mud crabs and other consumers, thus relieving predation on native species and enhancing recruitment or survival of bivalves and oyster drills. In contrast, the invasive crab can consume crustacean larvae and via this feeding may suppress recruitment of native mud crabs. Our findings should be conservative given the low densities of P. armatus seeded into experimental plots and our inability to run longer-term experiments due to controls rapidly being colonized by non-native crabs recruiting from the plankton. Invasive crabs commonly impact native communities via predation, but community impacts of this invasive crab may be as much due to its role as a preferred prey of native consumers as to its predation on native prey. Given that oysters are foundation species for shallow reefs in the South Atlantic Bight, the long-term effects of this invasion could be considerable.








Similar content being viewed by others
References
Brawley SH, Adey WH (1981) The effect of micrograzers on algal community structure in a coral reef microcosm. Marine Biol 61:167–177
Brown KM (1997) Size-specific aspects of the foraging ecology of the southern oyster drill Stramonita haemastoma (Kool 1987). J Exp Marine Biol Ecol 214:249–262
Caine EA (1975) Feeding and masticatory structures of selected Anomura (Crustacea). J Exp Mar Biol Ecol 18:277–301
Callaway RM, Aschehoug ET (2000) Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290:521–523
Carlton JT, Geller JB (1993) Ecological roulette: the global transport of nonindigenous marine organisms. Science 261:78–82
Elton C (1958) The ecology of invasions by animals and plants. Methuen, London
Grosholz ED (2002) Ecological and evolutionary consequences of coastal invasions. TREE 17:22–27
Gurevitch J, Padilla DK (2004) Are invasive species a major cause of extinctions? TREE 19:470–475
Hollebone AL, Hay ME (2007a) Population dynamics of the non-native crab Petrolisthes armatus invading the South Atlantic Bight at densities of thousands m−2. Mar Ecol Prog Ser 336:211–223
Hollebone AL, Hay ME (2007b) Propagule pressure of an invasive crab overwhelms biotic resistance. Mar Ecol Prog Ser (in press)
Juliano SA, Lounibos LP (2005) Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett 8:558–574
King RB, Ray JM, Stanford KM (2006) Gorging on gobies: beneficial effects of alien prey on a threatened vertebrate. Can J Zool 84:108–115
Knott D, Boyko C, Harvey A (2000) Introduction of the green porcelain crab, Petrolisthes armatus (Gibbes 1850), into the South Atlantic Bight. In: Pederson J (ed) Marine bioinvasions: Proceedings of the First National Conference, January 24–27, 1999. MIT Sea Grant College Program, Cambridge, MA
Kurlansky M (2006) The big oyster: history on the half shell. Ballantine Books, New York, NY
Lambrinos JG (2004) How interactions between ecology and evolution influence contemporary invasion dynamics. Ecology 85:2061–2070
Lee SY, Kneib RT (1994) Effects of biogenic structure on prey consumption by the xanthid crabs Eurytium limosum and Panopeus herbstii in a salt marsh. Mar Ecol Prog Ser 104:39–47
Lenihan HS, Peterson CH (1998) How habitat degradation through fishery disturbance enhances impacts of hypoxia on oyster reefs. Ecol Appl 8:128–140
Maerz JC, Karuzas JM, Madison DM, Blossey B (2005) Introduced invertebrates are important prey for a generalist predator. Div Distr 11:83–90
Migge-Klein S, McLean MA, Maerz JC, Heneghan L (2006) The influence of invasive earthworms on indigenous fauna in ecosystems previously uninhabited by earthworms. Biol Invasions 8:1275–1285
Mooney HA, Cleland EE (2001) The evolutionary impact of invasive species. Proc Nat Acad Sci 98:5446–5451
Nichols FH, Thompson JK, Schemel LE (1990) Remarkable invasion of San Francisco Bay (California, USA) by the Asian clam Potamocorbula amurensis II. Displacement of a former community. Mar Ecol Prog Ser 66:95–101
Oliveira E, Masunari S (1995) Estrutura populacional de Petrolisthes armatus (Gibbes) (Decapoda, Anomura, Porcellanidae) da Ilha do Farol, Matinhos, Parana, Brasil. Rev Brasil Zool 12:355–371
Parker JD, Burkepile DE, Hay ME (2006) Opposing effects of native and exotic herbivores on plant invasions. Science 311:1459–1461
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford
Rhymer JM, Simberloff D (1996) Extinction by hybridization and introgression. Annu Rev Ecol Syst 27:83–109
Rothschild BJ, Ault JS, Goulletquer P, Héral M (1994) Decline of the Chesapeake Bay oyster population: a century of habitat destruction and overfishing. Mar Ecol Prog Ser 111:29–39
Ruiz GM, Carlton JT, Grosholz ED, Hines AH (1997) Global invasions of marine and estuarine habitats by non-indigenous species: mechanisms, extent, and consequences. Am Zool 37:621–632
Sanders NJ, Gotelli NJ, Heller NE, Gordon DM (2003) Community disassembly by an invasive species. Proc Nat Acad Sci 100:2474–2477
Savidge JA (1987) Extinction of an island forest avifauna by an introduced snake. Ecology 68:660–668
Sax DF, Stachowicz JJ, Gaines SD (eds) (2005) Species invasions: insights into ecology, evolution, and biogeography. Sinauer Associates, Sunderland, MA
Seeley RH (1986) Intense natural selection caused a rapid morphological transition in a living marine snail. Proc Nat Acad Sci USA 83:6897–6901
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown. Biol Invasions 1:21–32
Stachowicz JJ, Fried H, Osman RW, Whitlach RB (2002) Biodiversity, invasion resistance, and marine ecosystem function: reconciling pattern and process. Ecology 83:2575–2590
Stanley JG, Sellers MA (1986) Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (Mid-Atlantic)-American Oyster. U. S. Fish and Wildlife Service Biological Report 82(11.65), US Army Corps of Engineers, TR EL-82–4
Trussell GC, Smith LD (2000) Induced defenses in response to an invading crab predator: an explanation of historical and geographic phenotypic change. Proc Nat Acad Sci USA 97:2123–2127
Vermeij GJ (2005) Invasion as expectation: a historical fact of life. In: Sax DF, Stachowicz JJ, Gaines SD (eds) Species invasions: insights into ecology, evolution, and biogeography. Sinauer, Sunderland, MA
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Upper Saddle River, NJ
Acknowledgments
We thank M. Ferner, C. Jordan, C. Kicklighter, J. Long, P. Munguia, C. Robertson, D. L. Smee, and the SkIO Facilities staff for assistance, K. Hollebone for artwork, and M. Miller, T. Snell, J. T. Streelman, and M. Weissburg for comments on the manuscript. Support was provided by EPA STAR (U−915531-01-0) and NOAA NERRS (NA03NOS4200063) Fellowships to A. L. H. with additional funding from the Harry and Linda Teasley Endowment to Georgia Tech.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hollebone, A.L., Hay, M.E. An invasive crab alters interaction webs in a marine community. Biol Invasions 10, 347–358 (2008). https://doi.org/10.1007/s10530-007-9134-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-007-9134-9