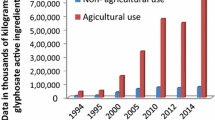
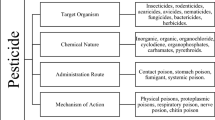
Abstract
Objective
To investigate the biochemical characterization of the carboxylesterase LmCesA1 from Locusta migratoria.
Results
We expressed recombinant LmCesA1 in Sf9 cells by using the Bac-to-bac baculovirus expression system. Enzyme kinetic assays showed that the Km values of LmCesA1 for α-naphthyl acetate (α-NA) and β-naphthyl acetate (β-NA) were 0.08 ± 0.01 mM and 0.22 ± 0.03 mM, respectively, suggesting that LmCesA1 has a higher affinity for α-NA. LmCesA1 retained its enzymatic activity during incubations at pH 7–10 and at 10–30 °C. In an inhibition experiment, two organophosphate pesticides (malaoxon and malathion) and one pyrethroid pesticide (deltamethrin) showed different inhibition profiles against purified LmCesA1. Recombinant LmCesA1 activity was significantly inhibited by malaoxon in vitro. UPLC analysis showed that no metabolites were detected.
Conclusions
These results suggest that overexpression of LmCesA1 enhances malathion sequestration to confer malathion tolerance in L. migratoria.






Similar content being viewed by others
References
Alon M, Alon F, Nauen R, Morin S (2008) Organophosphates’ resistance in the B-biotype of Bemisia tabaci (Hemiptera: Aleyrodidae) is associated with a point mutation in an ace1-type acetylcholinesterase and overexpression of carboxylesterase. Insect Biochem Mol Biol 38(10):940–949
Bai LS, Zhao CX, Xu JJ, Feng C, Li YQ, Dong YL, Ma ZQ (2019) Identification and biochemical characterization of carboxylesterase 001G associated with insecticide detoxification in Helicoverpa armigera. Pest Biochem Physiol 157:69–79
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Cai XH, Wang W, Lin L, He DH, Huang G, Shen YL, Wei W, Wei DZ (2017) Autotransporter domain-dependent enzymatic analysis of a novel extremely thermostable carboxylesterase with high biodegradability towards pyrethroid pesticides. Sci Rep 7:3461
Casida JE (1970) Mixed-function oxidase involvement in the biochemistry of insecticide synergists. J Agric Food Chem 18(5):753–772
Correy GJ, Zaidman D, Harmelin A, Carvalho S, Mabbitt PD, Calaora V, James PJ, Kotze AC, Jackson CJ, London N (2019) Overcoming insecticide resistance through computational inhibitor design. Proc Natl Acad Sci USA 116:21012–21021
Cuany A, Handani J, Berge J, Fournier D, Raymond M, Georghiou GP, Pasteur N (1993) Action of Esterase-B1 on chlorpyrifos in organophosphate-resistant Culex mosquitos. Pest Biochem Physiol 45(1):1–6
Cui F, Lin Z, Wang HS, Liu S, Chang HJ, Reeck G, Qiao CL, Raymond M, Kang L (2011) Two single mutations commonly cause qualitative change of nonspecific of nonspecific carboxylesterases in insects. Insect Biochem Mol Biol 41(1):1–8
Cui F, Qu H, Cong J, Liu XL, Qiao CL (2007) Do mosquitoes acquire organophosphate resistance by functional changes in carboxylesterases? FASEB J 21(13):3584–3591
Devonshire AL, Moores GD (1982) A carboxylesterase with broad substrate specificity causes organophosphorus, carbamate and pyrethroid resistance in peach-potato aphids (Myzus persicae). Pest Biochem Physiol 18(2):235–246
Feng XC, Li M, Liu NN (2018) Carboxylesterase genes in pyrethroid resistant house flies, Musca domestica. Insect Biochem Mol Biol 92:30–39
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Hopkins DH, Fraser NJ, Mabbitt PD, Carr PD, Oakeshott JG, Jackson CJ (2017) Structure of an insecticide sequestering carboxylesterase from the disease vector Culex quinquefasciatus: what makes an enzyme a good insecticide sponge? Biochemistry 56(41):5512–5525
Jackson CJ, Liu JW, Carr PD, Younus F, Coppin C, Meirelles T, Lethier M, Pandey G, Ollis DL, Russell RJ, Weik M, Oakeshott JG (2013) Structure and function of an insect α–carboxylesterase (αEsterase7) associated with insecticide resistance. Proc Natl Acad Sci USA 110(25):10177–10182
Jayawardena KGI, Karunaratne SHPP, Ketterman AJ, Hemingway J (1994) Determination of the role of elevated B2 esterase in insecticide resistance in Culex quinquefasciatus (Diptera: Culicidae) from studies on the purified enzyme. Bull Entomol Res 84(1):39–43
Ketterman AJ, Jayawardena KG, Hemingway J (1992) Purification and characterization of a carboxylesterase involved in insecticide resistance from the mosquito Culex quinquefasciatus. Biochem J 287(2):355–360
Lan WS, Cong J, Jiang H, Jiang SR, Qiao CL (2005) Expression and characterization of carboxylesterase E4 gene from peach-potato aphid (Myzus persicae) for degradation of carbaryl and malathion. Biotechnol Lett 27(15):1141–1146
Li YQ, Liu JW, Lu M, Ma ZQ, Cai CL, Wang YH, Zhang X (2016) Bacterial expression and kinetic analysis of carboxylesterase 001D from Helicoverpa armigera. Int J Mol Sci 17(4):493
Ma EB, He YP, Zhu KY (2004) Comparative studies of acetylcholinesterases purified from two field populations of the oriental migratory locust (Locusta migratoria manilensis): implications of insecticide resistance. Pest Biochem Physiol 78(1):67–77
Nováková L, Matysová L, Solich P (2006) Advantages of application of UPLC in pharmaceutical analysis. Talanta 68(3):908–918
Oakeshott JG, Claudianos C, Campbell PM, Newcomb RD, Russell RJ (2005) Biochemical genetics and genomics of insect esterases. Life Sci 5:309–381
Qin GH, Jia M, Liu T, Zhang XY, Guo YP, Zhu KY, Ma EB, Zhang JZ (2012) Heterologous expression and characterization of a sigma glutathione S-transferase involved in carbaryl detoxification from oriental migratory locust, Locusta migratoria manilensis (Meyen). J Insect Physiol 58(2):220–227
Swartz ME (2005) UPLC: an introduction and review. J Liq Chromatogr Relat Technol 28(7–8):1253–1263
Tang B, Dai W, Qi LJ, Du SK, Zhang CN (2020) Functional characterization of an α-esterase gene associated with malathion detoxification in Bradysia odoriphaga. J Agric Food Chem 68(22):6076–6083
U.S. Environmental Protection Agency, Office of pesticide programs (2016) Malathion: human health draft risk assessment for registration review, Federal register, pp 258
United States department of agriculture (2018) Draft human health and ecological risk assessment for malathion in exotic fruit fly applications, p 3
Wheelock CE, Shan GM, Ottea J (2005) Overview of Carboxylesterases and their role in the metabolism of insecticides. J Pestic Sci 30(2):75–83
Wu SW, Yang YH, Yuan GR, Campbell PM, Teese MG, Russell RJ, Oakeshott JG, Wu YD (2011) Overexpressed esterases in a fenvalerate resistant strain of the cotton bollworm, Helicoverpa armigera. Insect Biochem Mol Biol 41(1):14–21
Xie M, Ren NN, You YC, Chen WJ, Song QS, You MS (2017) Molecular characterization of two α-esterase genes involving chlorpyrifos detoxification in the diamondback moth, Plutella xylostella. Pest Manag Sci 73(6):1204–1212
Yang ML, Zhang JZ, Zhu KY, Xuan T, Liu XJ, Guo YP (2008) Mechanisms of organophosphate resistance in a field population of oriental migratory locust, Locusta migratoria manilensis (Meyen). Arch Insect Biochem Physiol 71(1):3–15
Zhang JQ, Ge PT, Li DQ, GuoYP Zhu KY, Ma EB, Zhang JZ (2015) Two homologous carboxylesterase genes from Locusta migratoria with different tissue expression patterns and roles in insecticide detoxification. J Insect Physiol 77:1–8
Zhang JQ, Li DQ, Ge PT, Guo YP, Zhu KY, Ma EB, Zhang JZ (2014) Molecular and functional characterization of cDNAs putatively encoding carboxylesterases from the migratory locust, Locusta migratoria. PLoS ONE 9(4):e94809
Zhang JQ, Li DQ, Ge PT, Yang ML, GuoYP Zhu KY, Ma EB, Zhang JZ (2013) RNA interference revealed the roles of two carboxylesterase genes in insecticide detoxification in Locusta migratoria. Chemosphere 93(6):1207–1215
Zhang JZ, Zhang JQ, Yang ML, Jia QD, Guo YP, Ma EB, Zhu KY (2011) Genomics-based approaches to screening carboxylesterase-like genes potentially involved in malathion resistance in oriental migratory locust (Locusta migratoria manilensis). Pest Manag Sci 67(2):183–190
Zhou L, Fang SM, Huang K, Yu QY, Zhang Z (2015) Characterization of an epsilon-class glutathione S-transferase involved in tolerance in the silkworm larvae after long term exposure to insecticides. Ecotoxicol Environ Saf 120:20–26
Zou CS, Cao CW, Zhang GC, Wang ZY (2014) Purification, characterization, and sensitivity to pesticides of carboxylesterase from Dendrolimus superans (Lepidoptera: Lasiocampidae). J Insect Sci 14(260):1–6
Acknowledgments
This research was supported by the National Natural Science Foundation of China (International Cooperation and Exchange Programme, No. 30810103907), Programs of Applied Basic Research of Shanxi Province (No. 201601D202058) and 2016 Provincial Support National Research Foundation of Shanxi Province (No. 226546001). The authors are grateful to Prof. Yoonseong Park and Rupinder Singh at the Department of Entomology, Kansas State University, Manhattan, Kansas, USA, for critical reading of manuscript.
Supporting information
Supplementary Table 1—Standard curve of CarE activity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yin, F., Ma, W., Li, D. et al. Expression and kinetic analysis of carboxylesterase LmCesA1 from Locusta migratoria. Biotechnol Lett 43, 995–1004 (2021). https://doi.org/10.1007/s10529-021-03086-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-021-03086-1