Abstract
Objective
To identify and characterize a novel bacterial pyranose 2-oxidase (P2Ox) and investigate its potential use in lignin degradation applications.
Results
A new bacterial P2Ox (PaP2Ox) enzyme was identified in the lignocellulolytic bacterium Pantoea ananatis Sd-1. The PaP2Ox open reading frame was cloned, and the encoded protein was heterologously expressed in an Escherichia coli expression system. Unlike another reported bacterial P2Ox enzyme, the purified PaP2Ox exhibits a homotetrameric spatial conformation that is similar to fungal P2Oxs, with each subunit having a molecular mass of 65 kDa. The recombinant PaP2Ox exhibits maximum activity at 50 °C and pH 6.5 with d-glucose as its preferred substrate. In addition, this enzyme was shown to work in combination with bacterial laccase in lignin degradation.
Conclusions
The bacterial enzyme PaP2Ox has potential use in ligninolytic systems and shows promising value in industrial biotechnological applications.





Similar content being viewed by others
References
Abadulla E, Tzanov T, Costa S, Robra KH, Cavaco-Paulo A, Gübitz GM (2000) Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl Environ Microbiol 66:3357–3362
Ai MQ, Wang FF, Zhang YZ, Huang F (2014) Purification of pyranose oxidase from the white rot fungus Irpex lacteus and its cooperation with laccase in lignin degradation. Process Biochem 49:2191–2198
Bannwarth M, Bastian S, Heckmann-Pohl D, Giffhorn F, Schulz GE (2004) Crystal structure of pyranose 2-oxidase from the white-rot fungus Peniophora sp. Biochemistry 43:11683–11690
Daniel G, Volc J, Kubatova E (1994) Pyranose oxidase, a major source of H2O2 during wood degradation by Phanerochaete chrysosporium, Trametes versicolor, and Oudemansiella mucida. Appl Environ Microbiol 60:2524–2532
Ferreira P, Carro J, Serrano A, Martínez AT (2015) A survey of genes encoding H2O2-producing GMC oxidoreductases in 10 Polyporales genomes. Mycologia 107:1105–1119
Giffhorn F (2000) Fungal pyranose oxidases: occurrence, properties and biotechnical applications in carbohydrate chemistry. Appl Microbiol Biotechnol 54:727–740
Goswami P, Chinnadayyala SS, Chakraborty M, Kumar AK, Kakoti A (2013) An overview on alcohol oxidases and their potential applications. Appl Microbiol Biotechnol 97:4259–4275
Hallberg BM, Leitner C, Haltrich D, Divne C (2004) Crystal structure of the 270 kDa homotetrameric lignin-degrading enzyme pyranose 2-oxidase. J Mol Biol 341:781–796
Leitner C, Volc J, Haltrich D (2001) Purification and characterization of pyranose oxidase from the white rot fungus Trametes multicolor. Appl Environ Microbiol 67:3636–3644
Leonowicz A, Rogalski J, Jaszek M, Luterek J, Wojtas-Wasilewska M, Malarczyk E, Ginalska G, Fink-Boots M, Cho NS (1999) Cooperation of fungal laccase and glucose 1-oxidase in transformation of björkman lignin and some phenolic compounds. Holzforschung 53:376–380
Ma JS, Zhang KK, Liao HD, Hector SB et al (2016) Genomic and secretomic insight into lignocellulolytic system of an endophytic bacterium Pantoea ananatis Sd-1. Biotechnol Biofuels 9:1–15
Mendes S, Banha C, Madeira J, Santos D, Miranda V, Manzanera M, Ventura MR, Berkel W, Martins LO (2016) Characterization of a bacterial pyranose 2-oxidase from Arthrobacter siccitolerans. J Mol Catal B Enzym 133:S34–S43
Shi XW, Liu Q, Ma JS, Liao HD et al (2015) An acid-stable bacterial laccase identified from the endophyte Pantoea ananatis Sd-1 genome exhibiting lignin degradation and dye decolorization abilities. Biotechnol Lett 37:2279–2288
Takakura Y (2015) Tricholoma matsutake fruit bodies secrete hydrogen peroxide as a potent inhibitor of fungal growth. Can J Microbiol 61:447–450
Takakura Y, Kuwata S (2003) Purification, characterization, and molecular cloning of a pyranose oxidase from the fruit body of the basidiomycete, Tricholoma matsutake. Biosci Biotechnol Biochem 67:2598–2607
Vecerek B, Maresova H, Kocanova M, Kyslik P (2004) Molecular cloning and expression of the pyranose 2-oxidase cDNA from Trametes ochracea MB49 in Escherichia coli. Appl Microbiol Biotechnol 64:525–530
Vrielink A, Lloyd LF, Blow DM (1991) Crystal structure of cholesterol oxidase from Brevibacterium sterolicum refined at 1.8 Å resolution. J Mol Biol 219:533–554
Xiong XQ, Liao HD, Ma JS, Liu XM, Zhang LY, Shi XW, Yang XL, Lu XN, Zhu YH (2014) Isolation of a rice endophytic bacterium, Pantoea sp. Sd-1, with ligninolytic activity and characterization of its rice straw degradation ability. Lett Appl Microbiol 58:123–129
Acknowledgements
This research was supported by the National Natural Science Foundation of China (31672093 and 51378191).
Supporting information
Supplementary Fig. 1—Electrophoretic analysis of PaP2Ox before and after GST tag cleavage.
Supplementary Fig. 2— Fundamental enzymatic characteristics of the cleaved PaP2Ox.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10529_2018_2538_MOESM1_ESM.tif
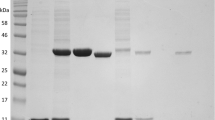
Supplementary material 1 (TIFF 20315 kb). Supplementary Fig. 1 Electrophoretic analysis of PaP2Ox before and after GST tag cleavage. Lane M, molecular mass marker; lane 1, supernatant of the sonicated extraction from E. coli BL21 (DE3) cells that overexpressed PaP2Ox-containing pGEX-4T-1; lane 2, purified recombinant GST-tagged PaP2Ox; lane 3, the supernatant containing cleaved PaP2Ox protein (65 kDa) and trace amounts of GST-PaP2Ox (91 kDa) and GST tag (26 kDa) after thrombin cleavage; lane 4, the elution mixture containing GST-PaP2Ox (91 kDa), cleaved PaP2Ox (65 kDa) and GST (26 kDa)
10529_2018_2538_MOESM2_ESM.tif
Supplementary material 2 (TIFF 41451 kb). Supplementary Fig. 2 Fundamental enzymatic characteristics of the cleaved PaP2Ox. The optimum temperature (a) of PaP2Ox was measured at pH 6.5. The thermostability (b) of PaP2Ox was analysed at pH 6.5 for 30 min, and the residual activity was measured under the standard assay conditions. The optimum pH (c) of PaP2Ox was measured at room temperature. The pH (d) stability of PaP2Ox was investigated at 4°C, and the residual activity was measured under the standard assay conditions
Rights and permissions
About this article
Cite this article
Zhang, K., Huang, M., Ma, J. et al. Identification and characterization of a novel bacterial pyranose 2-oxidase from the lignocellulolytic bacterium Pantoea ananatis Sd-1. Biotechnol Lett 40, 871–880 (2018). https://doi.org/10.1007/s10529-018-2538-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-018-2538-z