Abstract
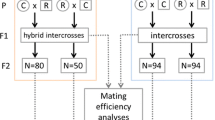
Successive rearing in laboratory conditions can result in the loss of genetic diversity, inbreeding depression and adaptation to the captive environment, affecting the quality of the insects reared and compromising their field performance. Introduction of genetic variation by admixing different populations may increase the fitness of populations, minimizing the negative effects of rearing many generations in artificial conditions. We experimentally investigated the role of intraspecific hybridization in enhancing the fitness of the egg parasitoid Trichogramma galloi Zucchi, 1988 (Hymenoptera: Trichogrammatidae), by reciprocally crossing three populations. Our results showed that the mating type did not affect the number of crosses that produced viable daughters. Homotypic crosses produced 94% viable daughters, while heterotypic crosses produced 92%. There were neither mating incompatibilities nor reproductive barriers between these populations. However, we observed a low fitness value for females from one of the populations studied. The fitness of hybrids was either unchanged or improved (in one case) when compared to the parental populations. We discuss the implications of our results and suggest future research directions.

Similar content being viewed by others
References
Andersen DH, Putridly C, Scali V, Loeschcke V (2002) Intraspecific hybridization, developmental stability and fitness in Drosophila mercatorum. Evol Ecol Res 4:603–621
Anderson E, Stebbins GL (1954) Hybridization as an evolutionary stimulus. Evolution 8:378–388
Antolin MF (1999) A genetic perspective on mating systems and sex ratios of parasitoid wasps. Res Popul Ecol 41:29–37
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Benvenuto C, Tabone E, Vercken E, Sorbier N, Colombel E, Warot S, Fauvergue X, Ris N (2012) Intraspecific variability in the parasitoid wasp Trichogramma chilonis: can we predict the outcome of hybridization? Evol Appl 5:498–510
Bertin A, Pavinato VAC, Parra JRP (2017) Fitness-related changes in laboratory populations of the egg parasitoid Trichogramma galloi and the implications of rearing on factitious hosts. BioControl 62:435–444
Braig HR, Zhou W, Dobson SL, O’Neil SL (1998) Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis. J Bacteriol 180:2373–2378
Burke JM, Arnold ML (2001) Genetics and the fitness of hybrids. Annu Rev Genet 35:31–52
Carvajal-Rodríguez A, Rolán-Alvarez E (2006) JMATING: a software for the analysis of sexual selection and sexual isolation effects from mating frequency data. BMC Evol Biol 6:40
Edmands S (2002) Does parental divergence predict reproductive compatibility? Trends Ecol Evol 17:520–527
Edmands S (2007) Between a rock and a hard place: evaluating the relative risks of inbreeding and outbreeding for conservation and management. Mol Ecol 16:463–475
Engelstädter J, Hurst GDD (2009) The ecology and evolution of microbes that manipulate host reproduction. Annu Rev Ecol Syst 40:127–149
Facon B, Pointier JP, Jarne P, Sarda V, David P (2008) High genetic variance in life-history strategies within invasive populations by way of multiple introductions. Curr Biol 18:363–367
Facon B, Crespin L, Loiseau A, Lombaert E, Magro A, Estoup A (2011) Can things get worse when an invasive species hybridizes? The harlequin ladybird Harmonia axyridis in France as a case study. Evol Appl 4:71–88
Frankham R (2008) Genetic adaptation to captivity in species conservation programs. Mol Ecol 17:325–333
Hassan SA (1993) The mass rearing and utilization of Trichogramma to control lepidopterous pests: achievements and outlook. Pestic Sci 37:387–391
Henter HJ (2003) Inbreeding depression and haplodiploidy: experimental measures in a parasitoid and comparisons across diploid and haplodiploid insect taxa. Evolution 57:1793–1803
Hopper KR, Roush RT, Powell W (1993) Management of genetics of biological control introductions. Annu Rev Entomol 38:27–51
Hoy MA (1976) Genetic improvement of insects: fact or fantasy. Environ Entomol 5:833–839
Johansen-Morris AD, Latta RG (2006) Fitness consequences of hybridization between ecotypes of Avena barbata: hybrid breakdown, hybrid vigor and transgressive segregation. Evolution 60:1585–1595
Lewontin RC, Birch LC (1966) Hybridization as a source of variation for adaptation to new environments. Evolution 20:315–336
Lommen STE, de Jong PW, Pannebakker BA (2017) It is time to bridge the gap between exploring and exploiting: prospects for utilizing intraspecific genetic variation to optimize arthropods for augmentative pest control—a review. Entomol Exp Appl 162:108–123
Lynch M, Walsh B (1998) Genetics and analysis of quantitative traits. Sinauer Associates, Sunderland
Mallet J (2005) Hybridization as an invasion of the genome. Trends Ecol Evol 20:229–237
Parra JRP (1997) Técnicas de criação de Anagasta kuehniella, hospedeiro alternativo para produção de Trichogramma. In: Parra JRP, Zucchi RA (eds) Trichogramma e o controle biológico aplicado. FEALQ, Piracicaba, pp 121–150
Parra JRP (2014) Biological control in Brazil: an overview. Sci Agric 71:420–429
Parra JRP, Zucchi AR (2004) Trichogramma in Brazil: feasibility of use after twenty years of research. Neotrop Entomol 33:271–281
Pekkala N, Knott KE, Kotiaho JS, Nissinen K, Puurtinen M (2012) The benefits of interpopulation hybridization diminish with increasing divergence of small populations. J Evol Biol 25:2181–2193
R Development Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.r-project.org/
Rius M, Darling JA (2014) How important is intraspecific genetic admixture to the success of colonising populations? Trends Ecol Evol 29:233–242
Rolán-Alvarez E, Caballero M (2000) Estimating sexual selection and sexual isolation effects from mating frequencies. Evolution 54:30–36
Saito Y, Sahara K, Mori K (2000) Inbreeding depression by recessive deleterious genes affecting female fecundity of a haplo-diploid mite. J Evol Biol 13:668–678
Seko T, Miyatake T, Miura K (2012) Assessment of hybrid vigor between flightless lines to restore survival and reproductive characteristics in the ladybird beetle Harmonia axyridis. BioControl 57:85–93
Stouthamer R, Luck RF, Hamilton WD (1990) Antibiotics cause parthenogenetic Trichogramma (Hymenoptera: Trichogrammatidae) to revert sex. Proc Natl Acad Sci USA 87:2424–2427
Stouthamer R, Breeuwer JAJ, Hurst GDD (1999) Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol 53:71–102
Szücs M, Eigenbrode SD, Schwarzländer M, Schaffner U (2012) Hybrid vigor in the biological control agent, Longitarsus jacobaeae. Evol Appl 5:489–497
Tien NSH, Sabelis MW, Egas M (2015) Inbreeding depression and purging in a haplodiploid: gender-related effects. Heredity 114:327–332
Vorsino AE, Wieczorek AM, Wright MG, Messing RH (2012) An analysis of heterosis and outbreeding depression among lab-reared populations of the parasitoid Diachasmimorpha tryoni (Cameron) (Hymenoptera: Braconidae); potential implications for augmentative releases. Biol Control 61:26–31
Waser NM, Price MV, Shaw RG (2000) Outbreeding depression varies among cohorts of Ipomopsis aggregata planted in nature. Evolution 54:485–491
Werren JH (1993) The evolution of inbreeding in a haplodiploid organism. In: Thornhill NW (ed) The natural history of inbreeding and outbreeding. University of Chicago Press, Chicago, pp 42–59
Whitlock MC, Ingvarsson PK, Hatfield T (2000) Local drift load and the heterosis of interconnected populations. Heredity 84:452–457
Woodworth LM, Montgomery ME, Briscoe DA, Frankham R (2002) Rapid genetic deterioration in captive populations: causes and conservation implications. Conserv Genet 3:277–288
Wright MG, Bennett GM (2018) Evolution of biological control agents following introduction to new environments. BioControl 63:105–116
Acknowledgements
We thank Dr. Janet W. Reid (JWR Associates) for English and technical corrections, the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (Processo: 140539/2012-3) for granting a scholarship to the first author, and the National Institute of Semiochemicals in Agriculture (Process nos. CNPq 573761/2008-6 and FAPESP 2008/57701-2) for financial support.
Funding
This study was funded by the National Institute of Semiochemicals in Agriculture (Process nos. CNPq 573761/2008-6 and FAPESP 2008/57701-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Stefano Colazza.
Rights and permissions
About this article
Cite this article
Bertin, A., Pavinato, V.A.C. & Parra, J.R.P. Effects of intraspecific hybridization on the fitness of the egg parasitoid Trichogramma galloi. BioControl 63, 555–563 (2018). https://doi.org/10.1007/s10526-018-9883-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-018-9883-7