Abstract
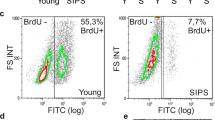
Senescent fibroblasts are characterized by their inability to proliferate and by a pro-inflammatory and catabolic secretory phenotype, which contributes to age-related pathologies. Furthermore, senescent fibroblasts when cultured under classical conditions in vitro are also characterized by striking morphological changes, i.e. they lose the youthful spindle-like appearance and become enlarged and flattened, while their nuclei from elliptical become oversized and highly lobulated. Knowing the strong relation between cell shape and function, we cultured human senescent fibroblasts on photolithographed Si/poly(vinyl alcohol) (PVA) micro-patterned surfaces in order to restore the classical spindle-like geometry and subsequently to investigate whether the changes in senescent cells’ morphology are the cause of their functional alterations. Interestingly, under these conditions senescent cells’ nuclei do not revert to the classical elliptical phenotype. Furthermore, enforced spindle-shaped senescent cells retained their deteriorated proliferative ability, and maintained the increased gene expression of the cell cycle inhibitors p16Ink4a and p21Waf1. In addition, Si/PVA-patterned-grown senescent fibroblasts preserved their senescence-associated phenotype, as evidenced by the overexpression of inflammatory and catabolic genes such as IL6, IL8, ICAM1 and MMP1 and MMP9 respectively, which was further manifested by an intense downregulation of fibroblasts’ most abundant extracellular matrix component Col1A, compared to their young counterparts. These data indicate that the restoration of the spindle-like shape in senescent human fibroblasts is not able to directly alter major functional traits and restore the youthful phenotype.








Similar content being viewed by others
References
Bourkoula A, Mavrogonatou E, Pavli P, Petrou PS, Douvas AM, Argitis P, Kletsas D, Kakabakos SE (2018) Guided cell adhesion, orientation, morphology and differentiation on silicon substrates photolithographically micropatterned with a cell-repellent cross-linked poly(vinyl alcohol) film. Biomed Mater 14(1):014101. https://doi.org/10.1088/1748-605X/aae7ba
Campisi J, d'Adda di Fagagna F (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8(9):729–740. https://doi.org/10.1038/nrm2233
Choi HR, Cho KA, Kang HT, Lee JB, Kaeberlein M, Suh Y, Chung IK, Park SC (2011) Restoration of senescent human diploid fibroblasts by modulation of the extracellular matrix. Aging Cell 10(1):148–157. https://doi.org/10.1111/j.1474-9726.2010.00654.x
Clause KC, Barker TH (2013) Extracellular matrix signaling in morphogenesis and repair. Curr Opin Biotechnol 24(5):830–833. https://doi.org/10.1016/j.copbio.2013.04.011
Collado M, Blasco MA, Serrano M (2007) Cellular senescence in cancer and aging. Cell 130(2):223–233. https://doi.org/10.1016/j.cell.2007.07.003
Coppe JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118. https://doi.org/10.1146/annurev-pathol-121808-102144
d'Adda di Fagagna F (2008) Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer 8(7):512–522. https://doi.org/10.1038/nrc2440
Freund A, Laberge RM, Demaria M, Campisi J (2012) Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell 23(11):2066–2075. https://doi.org/10.1091/mbc.E11-10-0884
Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M, Demaria M (2019) Cellular senescence: defining a path forward. Cell 179(4):813–827. https://doi.org/10.1016/j.cell.2019.10.005
Haupt A, Minc N (2018) How cells sense their own shape—mechanisms to probe cell geometry and their implications in cellular organization and function. J Cell Sci. https://doi.org/10.1242/jcs.214015
Hynes RO (2009) The extracellular matrix: not just pretty fibrils. Science 326(5957):1216–1219. https://doi.org/10.1126/science.1176009
Jeon H, Simon CG Jr, Kim G (2014) A mini-review: Cell response to microscale, nanoscale, and hierarchical patterning of surface structure. J Biomed Mater Res B 102(7):1580–1594. https://doi.org/10.1002/jbm.b.33158
Kilian KA, Bugarija B, Lahn BT, Mrksich M (2010) Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci USA 107(11):4872–4877. https://doi.org/10.1073/pnas.0903269107
Konstantonis D, Papadopoulou A, Makou M, Eliades T, Basdra EK, Kletsas D (2013) Senescent human periodontal ligament fibroblasts after replicative exhaustion or ionizing radiation have a decreased capacity towards osteoblastic differentiation. Biogerontology 14(6):741–751. https://doi.org/10.1007/s10522-013-9449-0
Li F, Li B, Wang QM, Wang JH (2008) Cell shape regulates collagen type I expression in human tendon fibroblasts. Cell Motil Cytoskelet 65(4):332–341. https://doi.org/10.1002/cm.20263
Liakou E, Mavrogonatou E, Pratsinis H, Rizou S, Evangelou K, Panagiotou PN, Karamanos NK, Gorgoulis VG, Kletsas D (2016) Ionizing radiation-mediated premature senescence and paracrine interactions with cancer cells enhance the expression of syndecan 1 in human breast stromal fibroblasts: the role of TGF-beta. Aging (Albany NY) 8(8):1650–1669. https://doi.org/10.18632/aging.100989
Linnane AW, Marzuki S, Ozawa T, Tanaka M (1989) Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet 1(8639):642–645. https://doi.org/10.1016/s0140-6736(89)92145-4
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Lukasova E, Kovarik A, Kozubek S (2018) Consequences of Lamin B1 and Lamin B receptor downregulation in senescence. Cells. https://doi.org/10.3390/cells7020011
Martinez E, Engel E, Planell JA, Samitier J (2009) Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann Anat 191(1):126–135. https://doi.org/10.1016/j.aanat.2008.05.006
Mavrogonatou E, Pratsinis H, Papadopoulou A, Karamanos NK, Kletsas D (2019) Extracellular matrix alterations in senescent cells and their significance in tissue homeostasis. Matrix Biol 75–76:27–42. https://doi.org/10.1016/j.matbio.2017.10.004
McFarland GA, Holliday R (1994) Retardation of the senescence of cultured human diploid fibroblasts by carnosine. Exp Cell Res 212(2):167–175. https://doi.org/10.1006/excr.1994.1132
Mehta IS, Figgitt M, Clements CS, Kill IR, Bridger JM (2007) Alterations to nuclear architecture and genome behavior in senescent cells. Ann N Y Acad Sci 1100:250–263. https://doi.org/10.1196/annals.1395.027
Papadopoulou A, Kletsas D (2011) Human lung fibroblasts prematurely senescent after exposure to ionizing radiation enhance the growth of malignant lung epithelial cells in vitro and in vivo. Int J Oncol 39(4):989–999. https://doi.org/10.3892/ijo.2011.1132
Papadopoulou A, Iliadi A, Eliades T, Kletsas D (2017) Early responses of human periodontal ligament fibroblasts to cyclic and static mechanical stretching. Eur J Orthod 39(3):258–263. https://doi.org/10.1093/ejo/cjw075
Pavli P, Petrou PS, Douvas AM, Dimotikali D, Kakabakos SE, Argitis P (2014) Protein-resistant cross-linked poly(vinyl alcohol) micropatterns via photolithography using removable polyoxometalate photocatalyst. ACS Appl Mater Interfaces 6(20):17463–17473. https://doi.org/10.1021/am5053224
Rattan SI, Clark BF (1994) Kinetin delays the onset of ageing characteristics in human fibroblasts. Biochem Biophys Res Commun 201(2):665–672. https://doi.org/10.1006/bbrc.1994.1752
Shay JW, Wright WE (2005) Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis 26(5):867–874. https://doi.org/10.1093/carcin/bgh296
Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK (2016) Extracellular matrix structure. Adv Drug Deliv Rev 97:4–27. https://doi.org/10.1016/j.addr.2015.11.001
Thery M (2010) Micropatterning as a tool to decipher cell morphogenesis and functions. J Cell Sci 123(Pt 24):4201–4213. https://doi.org/10.1242/jcs.075150
Toussaint O, Medrano EE, von Zglinicki T (2000) Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol 35(8):927–945. https://doi.org/10.1016/s0531-5565(00)00180-7
Wan LQ, Kang SM, Eng G, Grayson WL, Lu XL, Huo B, Gimble J, Guo XE, Mow VC, Vunjak-Novakovic G (2010) Geometric control of human stem cell morphology and differentiation. Integr Biol (Camb) 2(7–8):346–353. https://doi.org/10.1039/c0ib00016g
Zhong Y, Ji B (2013) Impact of cell shape on cell migration behavior on elastic substrate. Biofabrication 5(1):015011. https://doi.org/10.1088/1758-5082/5/1/015011
Funding
This work was supported by the project “Target Identification and Development of Novel Approaches for Health and Environmental Applications” (MIS 5002514), which is implemented under the Action for the Strategic Development on the Research and Technological Sectors, funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020) and co-financed by Greece and the European Union (European Regional Development Fund).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to the authorship and/or the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Papadopoulou, A., Kanioura, A., Petrou, P.S. et al. Reacquisition of a spindle cell shape does not lead to the restoration of a youthful state in senescent human skin fibroblasts. Biogerontology 21, 695–708 (2020). https://doi.org/10.1007/s10522-020-09886-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-020-09886-8