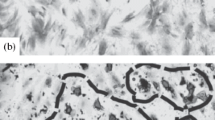
Changes in the muscular tissue after subcutaneous injection of autologous bone marrow multipotent mesenchymal stromal cells transfected with GFP gene and additionally stained with cell membrane dye Vybrant CM-Dil in the projection of ligated femoral vein were studied by light microscopy with luminescence. Stromal cells injected through the skin can appear not only in the damaged tissue where acceleration of regeneration processes is required, but also in intact structures located in superficial or deeper layers. In intact muscular tissue, stromal cells spreading in the perivascular tissue initiate inflammation and migration of macrophages, activate and even trigger sclerotic processes due to differentiation into connective tissue cells (fibroblasts) and stimulation of proliferation and collagen synthesis by host fibroblasts. Injected multipotent mesenchymal stromal cells are gradually phagocytized by macrophages.
Similar content being viewed by others
References
Maiborodin IV, Matveyeva VA, Maslov RV, Onopriyenko NV, Kuznetsova IV, Chastikin GA, Anikeyev AA. Some reactions of the regional lymph nodes of rats after implantation of multipotent stromal cells adsorbed on polyhydroxyalkanoate into a bone tissue defect. Morfologiya. 2016;149(2):21-26. Russian.
Maiborodin IV, Morozov VV, Matveeva VA, Anikeev AA, Figurenko NF, Maslov RV, Chastikin GA, Maiborodina VI3. Results of Experimental Ligation of the Main Vein with the Use of Cell Technologies. Bull. Exp. Biol. Med. 2017;164(1):61-67.
Chai NL, Zhang XB, Chen SW, Fan KX, Linghu EQ. Umbilical cord-derived mesenchymal stem cells alleviate liver fibrosis in rats. World J. Gastroenterol. 2016;22(26):6036-6048.
Haldar D, Henderson NC, Hirschfield G, Newsome PN. Mesenchymal stromal cells and liver fibrosis: a complicated relationship. FASEB J. 2016;30(12):3905-3928.
Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J. Clin. Invest. 2015;125(10):3992. doi: https://doi.org/10.1172/JCI84508.
Liu J, Hsu A, Lee JF, Cramer DE, Lee MJ. To stay or to leave: Stem cells and progenitor cells navigating the S1P gradient. World J. Biol. Chem. 2011;2(1):1-13.
Liu S, Jiang L, Li H, Shi H, Luo H, Zhang Y, Yu C, Jin Y. Mesenchymal stem cells prevent hypertrophic scar formation via inflammatory regulation when undergoing apoptosis. J. Invest. Dermatol. 2014;134(10):2648-2657.
Marycz K, Krzak J, Marędziak M, Tomaszewski KA, Szczurek A, Moszak K. The influence of metal-based biomaterials functionalized with sphingosine-1-phosphate on the cellular response and osteogenic differentaion potenial of human adipose derived mesenchymal stem cells in vitro. J. Biomater. Appl. 2016;30(10):1517-1533.
Mitchell AJ, Pradel LC, Chasson L, Van Rooijen N, Grau GE, Hunt NH, Chimini G. Technical advance: autofluorescence as a tool for myeloid cell analysis. J. Leukoc. Biol. 2010;88(3):597-603.
Molenaar R, Greuter M, van der Marel AP, Roozendaal R, Martin SF, Edele F, Huehn J, Förster R, O’Toole T, Jansen W, Eestermans IL, Kraal G, Mebius RE. Lymph node stromal cells support dendritic cell-induced gut-homing of T cells. J. Immunol. 2009;183(10):6395-6402.
Poncelet AJ, Denis D, Gianello P. Cellular xenotransplantation. Curr. Opin. Organ Transplant. 2009;14(2):168-174.
van den Bogaerdt AJ, van der Veen VC, van Zuijlen PP, Reijnen L, Verkerk M, Bank RA, Middelkoop E, Ulrich MM. Collagen cross-linking by adipose-derived mesenchymal stromal cells and scar-derived mesenchymal cells: are mesenchymal stromal cells involved in scar formation? Wound Repair Regen. 2009;17(4):548-558.
Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Gronthos S, Wang S, Shi S. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res. Ther. 2010;1(1):5. doi: https://doi.org/10.1186/scrt5.
Yates CC, Nuschke A, Rodrigues M, Whaley D, Dechant JJ, Taylor DP, Wells A. Improved transplanted stem cell survival in a polymer gel supplemented with tenascin C accelerates healing and reduces scarring of murine skin wounds. Cell Transplant. 2017;26(1):103-113.
Zhao XF, Wang DL, Wei ZR, Xue QY, Yu LM. The research of fibroblasts from human hypertrophic scar showing a mesenchymal stem cell phenotype and multilineage differentiation potentialities. Zhonghua Zheng Xing Wai Ke Za Zhi. 2013;29(4):273-279.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Kletochnye Tekhnologii v Biologii i Meditsine, No. 4, pp. 241-248, October, 2017
Rights and permissions
About this article
Cite this article
Maiborodin, I.V., Morozov, V.V., Anikeev, A.A. et al. Some Peculiarities of Local Distribution of Multipotent Mesenchymal Stromal Cells after Their Injection into Intact Muscle Tissue in Experiment. Bull Exp Biol Med 164, 554–560 (2018). https://doi.org/10.1007/s10517-018-4031-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-018-4031-z