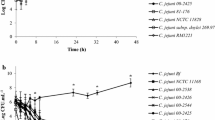
Specific features for the development of resistance in Campylobacter jejuni strains were studied after treatment with antibiotics of 6 pharmacological groups. Populations of 18 native strains of C. jejuni (isolated from raw poultry products) and their subcultures (obtained after 2-3-fold stress exposures to antimicrobial agents in subinhibitory doses) were examined to evaluate the expression of phenotypic antibiotic resistance. Genotypic properties of strains were studied by the PCR with primers that detect the presence of genes for resistance to aminoglycosides (aphA-1, aphA-3, and aphA-7), tetracyclines (tetO), and quinolones (GZgyrA). The majority of test strains of C. jejuni exhibited a high resistance to nalidixic acid, ciprofloxacin, and tetracycline, which reached the maximum value after numerous passages. The expression of antibiotic resistance was greatest in the presence of nalidixic acid and tetracycline. Ciprofloxacin resistance of 33% strains, which were initially resistant to this antibiotic, was increased after 2-3-fold treatment. We revealed a high degree of correspondence between phenotypic and genotypic profiles of antibiotic resistance in food isolates of Campylobacter. One, two, or more genes of aphA were identified in 85% strains phenotypically resistant to aminoglycosides. The tetO gene was found nearly in all strains resistant to tetracycline. Studying the biofilm matrix in C. jejuni after culturing with antibiotics in subinhibitory doses showed that quinolones (particularly nalidixic acid) and tetracyclines potentiate the formation of biofilms and increase the tolerance of Campylobacter to stress exposures. The intensity of biofilm growth was shown to depend little on the effect of macrolides and aminoglycosides. Therefore, the presence of these agents in residual concentrations is associated with a lower risk for the development of antibiotic resistance in C. jejuni populations.
Similar content being viewed by others
References
Efimochkina NR. Microbiology of Foodstuff and Modern Methods of Detection of Pathogens. Moscow, 2013. Russian.
Efimochkina NR, Bykova IB, Markova YM, Korotkevich YV, Stetsenko VV, Minaeva LP, Sheveleva SA. Formation of Biofilms by Foodborne Pathogens and Development of Laboratory In Vitro Model for the Study of Campylobacter Genus Bacteria Based on These Biofilms. Bull. Exp. Biol. Med. 2017;162(4):474-478.
Efimochkina NR, Korotkevich YuV, Stetsenko VV, Pichugina TV, Bykova IB, Markova YuM, Minaeva LP, Sheveleva SA. Antibiotic resistance of Campylobacter jejuni strains isolated from food products. Vopr. Pitaniya. 2017;(1):17-27. Russian.
Chebotar IV, Mayansky АN, Konchakova ЕD, Lazareva АV, Chistyakova VP. Antimicrobial Resistance of Bacteria in Biofilms. Klin. Mikrobiol. Antimikrob. Antimikrob. Khimioter. 2012;14(1):51-58. Russian.
Alfredson DA, Korolik V. Antibiotic resistance and resistance mechanisms in Campylobacter jejuni and Campylobacter coli. FEMS Microbiol. Lett. 2007;277(2):123-132.
Bae J, Oh E, Jeon B. Enhanced transmission of antibiotic resistance in Campylobacter jejuni biofilms by natural transformation. Antimicrob. Agents Chemother. 2014;58(12):7573-7575.
Gangaiah D, Kassem II, Liu Z, Rajashekara G. Importance of polyphosphate kinase 1 for Campylobacter jejuni viable-but-nonculturable cell formation, natural transformation, and antimicrobial resistance. Appl. Environ. Microbiol. 2009;75(24):7838-7849.
Nachamkin I, Guerry P. Campylobacter infections. Foodborne Pathogens: Microbiology and Molecular Biology. Fratamico PM, Bhunia AK, Smith JL, eds. Wymondham, 2005. P. 285-293.
Wang Y, Dong Y, Deng F, Liu D, Yao H, Zhang Q, Shen J, Liu Z, Gao Y, Wu C, Shen Z. Species shift and multidrug resistance of Campylobacter from chicken and swine, China, 2008-14. J. Antimicrob. Chemother. 2016;71(3):666-669.
Wirz ES, Overesch G, Kuhnert P, Korczak BM. Genotype and antibiotic resistance analyses of Campylobacter isolates from ceca and carcasses of slaughtered broiler flocks. Appl. Environ. Microbiol. 2010;76(19):6377-6386.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 164, No. 10, pp. 464-472, October, 2017
Rights and permissions
About this article
Cite this article
Efimochkina, N.R., Stetsenko, V.V., Bykova, I.V. et al. Studying the Phenotypic and Genotypic Expression of Antibiotic Resistance in Campylobacter jejuni under Stress Conditions. Bull Exp Biol Med 164, 466–472 (2018). https://doi.org/10.1007/s10517-018-4014-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-018-4014-0