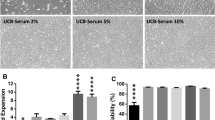
Optimal conditions for culturing of multipotent mesenchymal stromal cells in the presence of pooled umbilical cord blood serum were determined. It was found that umbilical cord blood serum in a concentration range of 1-10% effectively supported high viability and proliferative activity of cells with unaltered phenotype and preserved multilineage differentiation capacity. The proposed approach allows avoiding the use of xenogenic animal sera for culturing of multipotent mesenchymal stromal cells and creates prerequisites for designing and manufacturing safe cellular and/or acellular products for medical purposes.
Similar content being viewed by others
References
Caplan AI. Adult mesenchymal stem cells: when, where, and how. Stem Cells Int. 2015;2015. ID 628767. doi: 10.1155/2015/628767.
Castrén E, Sillat T, Oja S, Noro A, Laitinen A, Konttinen YT, Lehenkari P, Hukkanen M, Korhonen M. Osteogenic differentiation of mesenchymal stromal cells in two-dimensional and three-dimensional cultures without animal serum. Stem Cell Res. Ther. 2015;6:167. doi: 10.1186/s13287-015-0162-6.
Corotchi MC, Popa MA, Remes A, Sima LE, Gussi I, Lupu Plesu M. Isolation method and xeno-free culture conditions influence multipotent differentiation capacity of human Wharton’s jelly-derived mesenchymal stem cells. Stem Cell Res. Ther. 2013;4(4):81. doi: 10.1186/scrt232.
Díez JM, Bauman E, Gajardo R, Jorquera JI. Culture of human mesenchymal stem cells using a candidate pharmaceutical grade xeno-free cell culture supplement derived from industrial human plasma pools. Stem Cell Res. Ther. 2015;6:28: doi: 10.1186/s13287-015-0016-2.
Hervy M, Weber JL, Pecheul M, Dolley-Sonneville P, Henry D, Zhou Y, Melkoumian Z. Long term expansion of bone marrow-derived hMSCs on novel synthetic microcarriers in xeno-free, defined conditions. PLoS One. 2014;9(3):e92120.
Jung S, Panchalingam KM, Wuerth RD, Rosenberg L, Behie LA. Large-scale production of human mesenchymal stem cells for clinical applications. Biotechnol. Appl. Biochem. 2012;59(2):106-120.
Kim N, Cho SG. Clinical applications of mesenchymal stem cells. Korean J. Intern. Med. 2013;28(4):387-402.
Konala V.B, Mamidi MK, Bhonde R, Das AK, Pochampally R, Pal R. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy. 2016;18(1):13-24.
Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, Zhao GB, Ma ZJ. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015;6:55. doi: 10.1186/s13287-015-0066-5.
Liu Y, Li YQ, Wang HY, Li YJ, Liu GY, Xu X, Wu XB, Jing YG, Yao Y, Wu CT, Jin JD. Effect of serum choice on replicative senescence in mesenchymal stromal cells. Cytotherapy. 2015;17(7):874-884.
Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216-225.
Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013;45:e54. doi: 10.1038/emm.2013.94.
Oikonomopoulos A, van Deen WK, Manansala AR, Lacey PN, Tomakili TA, Ziman A, Hommes DW. Optimization of human mesenchymal stem cell manufacturing: the effects of animal/xeno-free media. Sci. Rep. 2015;5. ID 16570. doi: 10.1038/srep16570.
Riordan NH, Madrigal M, Reneau J, de Cupeiro K, Jiménez N, Ruiz S, Sanchez N, Ichim TE, Silva F, Patel AN. Scalable efficient expansion of mesenchymal stem cells in xeno free media using commercially available reagents. J. Transl Med. 2015;13:232.
Romanov YA, Balashova EE, Volgina NE, Kabaeva NV, Dugina TN, Sukhikh GT. Optimized protocol for isolation of multipotent mesenchymal stromal cells from human umbilical cord. Bull. Exp. Biol. Med. 2015;160(1):148-154.
Romanov YA, Darevskaya AN, Merzlikina NV, Buravkova LB. Mesenchymal stem cells from human bone marrow and adipose tissue: isolation, characterization, and differentiation potentialities. Bull. Exp. Biol. Med. 2005;140(1):138-143.
Stoltz JF, de Isla N, Li YP, Bensoussan D, Zhang L, Huselstein C, Chen Y, Decot V, Magdalou J, Li N, Reppel L, He Y. Stem cells and regenerative medicine: myth or reality of the 21th century. Stem Cells Int. 2015;2015. ID 734731. doi: 10.1155/2015/734731.
Suchánková Kleplová T, Soukup T, Řeháček V, Suchánek J. Human plasma and human platelet-rich plasma as a substitute for fetal calf serum during long-term cultivation of mesenchymal dental pulp stem cells. Acta Medica (Hradec Kralove). 2014;57(3):119-126.
Swamynathan P, Venugopal P, Kannan S, Thej C, Kolkundar U, Bhagwat S, Ta M, Majumdar AS, Balasubramanian S. Are serum-free and xeno-free culture conditions ideal for large scale clinical grade expansion of Wharton’s jelly derived mesenchymal stem cells? A comparative study. Stem Cell Res. Ther. 2014;5(4):88. doi: 10.1186/scrt477.
Venugopal P, Balasubramanian S, Majumdar AS, Ta M. Isolation, characterization, and gene expression analysis of Wharton’s jelly-derived mesenchymal stem cells under xeno-free culture conditions. Stem Cells Cloning. 2011;4:39-50.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Kletochnye Tekhnologii v Biologii i Meditsine, No. 4, pp. 215-220, October, 2016
Rights and permissions
About this article
Cite this article
Romanov, Y.A., Balashova, E.E., Volgina, N.E. et al. Human Umbilical Cord Blood Serum: Effective Substitute of Fetal Bovine Serum for Culturing of Human Multipotent Mesenchymal Stromal Cells. Bull Exp Biol Med 162, 528–533 (2017). https://doi.org/10.1007/s10517-017-3654-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-017-3654-9