Abstract
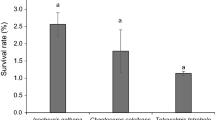
The nutritional value of nauplii and metanauplii I of Artemia enriched with microalgae as food for freshwater prawn larvae (Macrobrachium americanum) was tested. The larvae were fed with three different diets consisting of Artemia nauplii (D1), Artemia metanauplii I enriched with Tetraselmis suecica (D2), and Artemia metanauplii I enriched with Chaetoceros calcitrans (D3) from zoea (Z)II. Growth showed differences since the third week, and the highest and lowest growth was observed with D3 and D1, respectively. The first metamorphosis to post-larvae appeared with treatment D3 at 9 weeks. Survival showed differences at the first week with D1 and D3 treatments, and D2 showed the best survival up to week 4. From the sixth week to the end, treatment D3 reached the highest survival. With treatments D1 and D2, all larvae died at the 9th and 10th week, respectively. D3 was the most effective of the three diets.
Similar content being viewed by others
Abbreviations
- Z:
-
Zoea
- PUFAs:
-
Polyunsaturated fatty acids
- AA:
-
Arachidonic acid
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentanoic acid
- HUFAs:
-
Highly unsaturated fatty acids
- MUFAs:
-
Monounsaturated fatty acids
- LNA:
-
Linolenic acid
- LOA:
-
Linoleic acid
- D1 :
-
Artemia nauplii
- D2 :
-
Artemia metanauplii I enriched with Tetraselmis suecica
- D3 :
-
Artemia metanauplii I enriched with Chaetoceros calcitrans
References
Agard JB (1999) A four-dimensional response surface analysis of the ontogeny of physiological adaptation to salinity and temperature in larvae of the palaemonid shrimp Macrobrachium rosenbergii (de Man). J Exp Mar Biol Ecol 236:209–233
Ali SSR, Ambasankar K, Praveena PE, Nandakumar S, Syamadayal J (2017) Effect of dietary fructooligosaccharide supplementation on growth, body composition, hematological and immunological parameters of Asian seabass (Lates calcarifer). Aquac Int 25:837–848
Anger K, Hayd L (2009) From lecithotrophy to planktotrophy: ontogeny of larval feeding in the Amazon River prawn Macrobrachium amazonicum. Aquat Biol 7:19–30
Anh NTN, Hien TTT, Mathieu W, Hoa NV, Sorgeloos P (2009) Effect of fishmeal replacement with Artemia biomass as a protein source in practical diets for the giant freshwater prawn Macrobrachium rosenbergii. Aquac Res 40:669–680
Arana-Magallón MF (1974) Experiencias sobre el cultivo del langostino Macrobrachium americanum Bate, en el noroeste de México. In: Dupree HK, Price KS Jr, Shaw WN, Danberg KS (eds) Actas del Simposio sobre Acuicultura en América Latina, Vol 1. Documentos de investigación. FAO, Rome, pp 139–147
Araujo MC, Valenti WC (2007) Feeding habit of the Amazon river prawn Macrobrachium amazonicum larvae. Aquaculture 265:187–193
Bauer RT (2013) Amphidromy in shrimps: a life cycle between rivers and the sea. Lat Am J Aquat Res 41:633–650
Bell MV, Batty RS, Dick JR, Fretwell K, Navarro JC, Sargent JR (1995) Dietary deficiency of docosahexaenoic acid impairs vision at low light intensities in juvenile herring (Clupea harengus L.) Lipids 30:443–449
Benítez-Mandujano MA, Ponce-Palafox JT (2014) Effects of different dietary of protein and lipid levels on the growth of freshwater prawns (Macrobrachium carcinus) broodstock. Rev MVZ Córdoba 19:3921–3929
Bhavan PS, Devi VG, Shanti R, Radhakrishnan S, Poongodi R (2010) Basic biochemical constituents and profiles of amino acids in the post larvae of Macrobrachium rosenbergii fed with Spirulina and yeast enriched Artemia. J Sci Res 2:539–540
Boudour-Boucheker N, Boulo V, Charmantier-Daures M, Anger K, Charmantier G, Lorin-Nebel C (2016) Osmoregulation in larvae and juveniles of two recently separated Macrobrachium species: expression patterns of ion transporter genes. Comp Biochem Physiol A Mol Integr Physiol 195:39–45
Brett M, Müller-Navarra D (1997) The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshw Biol 38:483–499
Brown MF (1994) Modulation of rhodopsin function by properties of the membrane bilayer. Chem Phys Lipids 73:159–180
Castro J, Castro T, Sánchez J, Castro G, Castro A, Zaragoza J, Monroy MDC (2006) Cysts and nauplii biometry characteristics of seven Artemia franciscana (Kellog, 1906) populations from Mexico. Rev Biol Mar Oceanogr 41:187–193
Cavalli RO, Lavens P, Sorgeloos P (1999) Performance of Macrobrachium rosenbergii broodstock fed diets with different fatty acid composition. Aquaculture 179:387–402
Chakraborty RD, Chakraborty K, Radhakrishnan EV (2007) Variation in fatty acid composition of Artemia salina nauplii enriched with microalgae and baker's yeast for use in larviculture. J Agric Food Chem 55:4043–4051
Cho SH, Hur SB, Jo JY (2001) Effect of enriched live feeds on survival and growth rates in larval Korean rockfish, Sebastes schlegeli Hilgendorf. Aquac Res 32:199–208
Choudhury PC (1970) Complete larval development of the palaemonid shrimp Macrobrachium acanthurus (Wiegmann, 1836), reared in the laboratory. Crustaceana 18:113–132
Choudhury PC (1971) Complete larval development of the palaemonid shrimp Macrobrachium carcinus (L.), reared in the laboratory (Decapoda, Palaemonidae). Crustaceana 20:51–69
Daniels WH, D’Abramo LR, Parseval LD (1992) Design and management of a close recirculated clearwater hatchery system for freshwater Macrobrachium praws. J Shellfish Res 11:65–73
Das SK, Tiwari VK, Venkateshwarlu G, Reddy AK, Parhi J, Sharma P, Chettri JK (2007) Growth, survival and fatty acid composition of Macrobrachium rosenbergii (de Man, 1879) post larvae fed HUFA-enriched Moina micrura. Aquaculture 269:464–475
De Barros HP, Valenti WC (2003) Food intake of Macrobrachium rosenbergii during larval development. Aquaculture 216:165–176
De Grave S, Cai Y, Anker A (2008) Global diversity of shrimps (Crustacea: Decapoda: Caridea) in freshwater. Hydrobiologia 595:287–293
Devresse B, Romdhane MS, Buzzi M, Rasowo J, Léger P, Brown J, Sorgeloos P (1990) Improved larviculture outputs in the giant freshwater prawn Macrobrachium rosenbergii fed a diet of Artemia enriched with n-3 HUFA and phospholipids. J World Aquacult Soc 21:123–125
Dhont J, Van Stappen G (2003) Biology, tank production and nutritional value of Artemia. In: Josianne G, Støttrupm Lesley a (eds) Live feeds in marine aquaculture. McEvoy by Blackwell Science Ltd a Blackwell, pp 65–121
Folch J, Less M, Stanley GHS (1956) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Gamboa-Delgado J, Le Vay L (2009) Artemia replacement in co-feeding regimes for mysis and postlarval stages of Litopenaeus vannamei: nutritional contribution of inert diets to tissue growth as indicated by natural carbon stable isotopes. Aquaculture 297:128–135
García-Guerrero M, de los Santos-Romero R, Vega-Villasante F, Cortes-Jacinto E (2015) Conservation and aquaculture of native freshwater prawns: the case of the cauque river prawn Macrobrachium americanum (Bate, 1868). Lat Am J Aquat Res 43:819-827 https://doi.org/10.3856/vol43-issue5-fulltext-2
García-Guerrero M, Hendrickx ME (2009) External description of the embryonic development of the prawn, Macrobrachium americanum Bate, 1868 (Decapoda, Palaemonidae) based on the staging method. Crustaceana 82:1413–1422
García-Guerrero M, Orduña-Rojas J, Cortés-Jacinto E (2011) Size and temperature effects on oxygen consumption of the river prawn Macrobrachium americanum Bate over its regular temperature range. N Am J Aquac 73:320–326
Guillard RR, Lorenzen CJ (1972) Yellow-green algae with chlorophyllide C. J Phycol 8:10–14
Karuppasamy PK, Sri Sakthi Priyadarshini R, Ramamoorthy N, Sujatha R, Ganga S, Jayalakshmi T, Santhanam P (2014) Comparison of proximate, amino and fatty acid composition of Penaeus monodon (Fabricius, 1798), Fenneropenaeus indicus (H. Milne Edwards, 1837) and Aristeus virilis (Bate, 1881) of Nagapattinam landing Centre, Tamil Nadu. J Mar Biol Assoc India 55:5–10
Kotrbacek V, Doubek J, Doucha J (2015) The chlorococcalean alga Chlorella in animal nutrition: a review. J Appl Phycol 27:2173–2180
Lavens P, Sorgeloos P (2000) The history, present status and prospects of the availability of Artemia cysts for aquaculture. Aquaculture 181:397–403
Lavens P, Thongrod S, Sorgeloos P (2000) Larval prawn feeds and the dietary importance of Artemia. In: New MB, Valenti WC (eds) Freshwater prawn culture: the farming of Macrobrachium rosenbergii. Wiley-Blackwell, Oxford, pp 91–111
Léger P, Bengston DA, Simpson KL, Sorgeloos P (1986) The use and nutritional value of Artemia as a food source. Oceanogr Mar Biol Annu Rev 24:521–623
Lober M, Zeng C (2009) Effect of microalgae concentration on larval survival, development and growth of an Australian strain of giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 289:95–100
Lora-Vilchis MC, Ruiz-Velasco-Cruz E, Reynoso-Granados T, Voltolina D (2004) Evaluation of five microalgae diets for juvenile pen shells Atrina maura. J World Aquacult Soc 35:232–236
Lubzens E, Gibson O, Zmora O, Sukenik A (1995) Potential advantages of frozen algae (Nannochloropsis sp.) for rotifer (Brachionus plicatilis) culture. Aquaculture 133:295–309
Maciel CR, New MB, Valenti WC (2012) The predation of Artemia nauplii by the larvae of the Amazon River prawn, Macrobrachium amazonicum (Heller, 1862), is affected by prey density, time of day, and ontogenetic development. J World Aquacult Soc 43:659–669
Mantellato F, Barbosa L (2005) Population structure and relative growth of freshwater prawn Macrobrachium brasiliense (Decapoda, Palaemonidae) from São Paulo State, Brazil. Acta Limnol Bras 17:245–255
Méndez-Martínez Y, Yamasaki-Granados S, García-Guerrero MU, Martínez-Córdova LR, Rivas-Vega ME, Arcos-Ortega FG, Cortés-Jacinto E (2017) Effect of dietary protein content on growth rate, survival and body composition of juvenile cauque river prawn, Macrobrachium americanum (Bate 1868). Aquac Res 48:741–751
Méndez-Martínez Y, García-Guerrero MU, Arcos-Ortega FG, Martínez-Córdova LR, Yamasaki-Granados S, Pérez-Rodríguez JC, Cortés-Jacinto E (2018) Effect of different ratios of dietary protein-energy on growth, body proximal composition, digestive enzyme activity, and hepatopancreas histology in Macrobrachium americanum (Bate, 1868) prawn juveniles. Aquaculture 485:1–11
Moller TH (1978) Feeding behaviour of larvae and postlarvae of Macrobrachium rosenbergii (De Man) (Crustacea: Palaemonidae). J Exp Mar Biol Ecol 35:251–258
Monaco G (1975) Laboratory rearing of larvae of the palaemonid shrimp Macrobrachium americanum (Bate). Aquaculture 6:169–375
Morales MC, Meruane J (2012) Larval condition indicators applied to the northern river shrimp Cryphiops caementarius (Molina, 1782), under condition of controlled cultivation. Lat Am J Aquat Res 40:730-742
Muller-Feuga A, Moal J, Kaas R (2003) The microalgae of aquaculture. In: Josianne G, Støttrupm Lesley A (eds) Live feeds in marine aquaculture. McEvoy by Blackwell Science Ltd a Blackwell, pp 206–252
Naegel LC (1999) Controlled production of Artemia biomass using an inert commercial diet, compared with the microalgae Chaetoceros. Aquac Eng 21:49–59
Nandlal S (2010) A new species for culture in the Pacific: evaluation of the potential of the indigenous Macrobrachium lar (Fabricius, 1798). In: Unpublished Doctor of Philosophy Thesis. University of the South Pacific, SuvaNavarro JC,
Nelson MM, Crear BJ, Nichols PD, Ritz DA (2004) Growth and lipid composition of phyllosomata of the southern rock lobster, Jasus edwardsii, fed enriched Artemia. Aquac Nutr 10:237–246
New MB (2005) Freshwater prawn farming: global status, recent research and a glance at the future. Aquac Res 36:210–230
Nguyen DT, Vangansbeke D, De Clercq P (2014) Solid artificial diets for the phytoseiid predator Amblyseius swirskii. Biol Control 59:719–727
O'Connor JD, Gilbert LI (1968) Aspects of lipid metabolism in crustaceans. Am Zool 8:529–539
Racotta IS, Palacios E, Hernández-Herrera R, Bonilla A, Pérez-Rostro CI, Ramirez JL (2004) Criteria for assesing larval and postlarval quality in white pacific shrimp (Litopenaeus vannamei, Boone 1931). Aquaculture 233:181–195
Reyes-Marchán R, Hidalgo-Mogollón A (2001) Producción en laboratorio de post larvas de Macrobrachium americanum (Decapoda: Palaemonidae (Camarón de río). Undergraduate Thesis to obtain the degree in Fishery Engineering. Universidad Nacional de Tumbes, Facultad de Ingeniería Pesquera, Escuela Académico Profesional de Ingeniería Pesquera. Tumbes, Perú
Rivero-Rodríguez S, Beaumont AR, Lora-Vilchis MC (2007) The effect of microalgal diets on growth, biochemical composition, and fatty acid profile of Crassostrea corteziensis (Hertlein) juveniles. Aquaculture 263:199–210
Roe JH, Bailey JM, Gray RR, Robinson JN (1961) Complete removal of glycogen from tissues by extraction with cold trichloroacetic acid solution. J Biol Chem 236:1244–1246
Ross LG, Beveridge MCM (1995) Is a better strategy necessary for development of native species for aquaculture? A Mexican case study. Aquacult Fish Manag 26:539–547
Ross LG, Martinez-Palacios CA, Morales EJ (2008) Developing native fish species for aquaculture: the interacting demands of biodiversity, sustainable aquaculture and livelihoods. Aquac Res 39:675–683
Roustaian P, Kamarudin MS, Omar H, Saad CR, Ahmad MH (1999) Changes in fatty acid profile during larval development of freshwater prawn Macrobrachium rosenbergii (De Man). Aquac Res 30:815–824
Roychoudhury P, Mukherjee M (2013) Role of algal mixture in food intake of Macrobrachium rosenbergii during larval development. Indian J Geo Mar Sci 42:647–652
Sandifer PA, Joseph JD (1976) Growth responses and fatty acid composition of juvenile prawns (Macrobrachium rosenbergii) fed a prepared ration augmented with shrimp head oil. Aquaculture 8:129–138
Stryer L (1995) Fatty acid metabolism. Biochemistry 3:469–493
Thinh LV, Renaud SM, Parry DL (1999) Evaluation of recently isolated Australian tropical microalgae for the enrichment of the dietary value of brine shrimp, Artemia nauplii. Aquaculture 170:161–173
Tziouveli V, Hall M, Smith GG (2012) Evaluation of lipid-enriched Artemia on the reproductive performance of the white-striped cleaner shrimp, Lysmata amboinensis. Aquac Int 20:201–211
Van Stappen G (1996) Introduction, biology and ecology of Artemia. In: Lavens P, Sorgeloos P (eds) Manual on the production and use of live food for aquaculture, Vol 361. FAO fisheries technical paper, FAO, Rome, pp 79-264
Vega-Villasante F, Martínez-López EA, Espinosa-Chaurand LD, Cortés-Lara MC, Nolasco-Soria H (2011) Growth and survival of prawn (Macrobrachium tenellum) in experimental cultures during summer and autumn in the tropical Mexican Pacific coast. Trop Subtrop Agroecosyt 14:581–588
Vega-Villasante F, García-Guerrero MU, Cortés-Jacinto E, Yamasaki-Granados S, Montoya-Martínez CE, Vargas-Ceballos MA, Chong-Carrillo O, Rubio-Padilla MA, Guzmán-Arroyo M, Carrillo-Farnés OV (2014) Los camarones de agua dulce del género Macrobrachium: biología, ecología y explotación. In: Cifuentes-Lemus JL, Cupul-Magaña FG (eds) Temas sobre investigaciones costeras. Universidad de Guadalajara, Jalisco, pp 273–315
Voltolina D, López-Elías JA (2002) Cultivos de apoyo para la acuacultura: Tendencias e innovaciones. In: Martínez-Córdova LR (ed) Camaronicultura, avances y tendencias. AGT Editores, Mexico pp 23-35
Wantanabe T (1993) Importance of docosahexaenoic acid in marine larval fish. J World Aquacult Soc 24:152–161
Watanabe T (1982) Lipid nutrition in fish. Comp Biochem Physiol A 73:3–15
Webster CD, Lovell RT (1990) Response of striped bass larvae fed brine shrimp from different sources containing different fatty acid compositions. Aquaculture 90:49–61
Yamasaki-Granados S, García-Guerrero M, Vega-Villasante F, Castellanos-León F, Cavalli RO, Cortés-Jacinto E (2013) Experimental culture of the river prawn Macrobrachium americanum larvae (Bate, 1868), with emphasis on feeding and stocking density effect on survival. Lat Am J Aquat Res 41:793–800
Zar JH (1984) Biostatistical analysis. 2nd edition. In: Prentice-Hall (ed) Englewood Cliffs, New Jersey, 718 pp
Acknowledgements
We thank P. Monsalvo, A. Greene, J. Garzón, R. Herrera, and G. Mendoza from CIBNOR for their technical assistance, and A. Álvarez, who provided advice for the development of the culture system. We thank G. Talamantes for his help with the catching of adult prawn specimens, and Ingrid Mascher Gramlich her editorial services. Funding was provided by CONACYT project CB2010/156252, 2014/227565, AMEXCID CTC/06038/14. Y.M.M. is a CONACYT fellowship recipient (grant 2021420084). M. Garcia-Guerrero thanks IPN EDI and COFAA programs for their financial support. E. Cortés-Jacinto is a fellow of CONACYT (sabbatical project 262236–2015-03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Méndez-Martínez, Y., García-Guerrero, M.U., Lora-Vilchis, M.C. et al. Nutritional effect of Artemia nauplii enriched with Tetraselmis suecica and Chaetoceros calcitrans microalgae on growth and survival on the river prawn Macrobrachium americanum larvae. Aquacult Int 26, 1001–1015 (2018). https://doi.org/10.1007/s10499-018-0264-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-018-0264-0