Abstract
Observations of surface seawater fugacity of carbon dioxide (fCO2) and pH were collected over a period of several days at French Frigate Shoals (FFS) in the Northwestern Hawaiian Islands (NWHI) in order to gain an understanding of the natural spatiotemporal variability of the marine inorganic carbon system in a pristine coral reef ecosystem. These data show clear island-to-open ocean gradients in fCO2 and total alkalinity that can be measured 10–20 km offshore, indicating that metabolic processes influence the CO2–carbonic acid system over large areas of ocean surrounding FFS and by implication the islands and atolls of the NWHI. The magnitude and extent of this spatial gradient may be driven by a combination of physical and biogeochemical processes including reef water residence time, hydrodynamic forcing of currents and tidal flow, and metabolic processes that occur both on the reef and within the lagoon.
Similar content being viewed by others
1 Introduction
Coral reefs are one of the most productive and biologically diverse ecosystems on the planet. Despite covering less than 0.1% of the earth’s surface (Smith 1978; Smith and Mackenzie 2016), the economic value of coral reefs is an estimated 29.8 billion dollars annually (Cesar et al. 2003). Approximately 655 million people live within 100 km of coral reefs, 91% of whom live in developing nations (Donner and Potere 2007), and many of these people depend directly on reef resources for survival. Disruptions to these critically important ecosystems could have cascading effects for ocean ecology, economic stability and human health.
One of the potential threats to coral reefs is ocean acidification (OA). OA refers to the oceanic absorption of anthropogenic carbon dioxide (CO2) and the consequent decline in ocean pH (Caldeira and Wickett 2003; Feely et al. 2004). Under the ‘business as usual” scenario RCP8.5, average open ocean surface seawater pH is expected to decline 0.33 units by the end of the twenty-first century (Bopp et al. 2013), and van Hooidonk et al. (2014) suggest that calcification at all reef locations worldwide will decline by 5% by the year 2034.
Although numerous experimental and field studies show that increasing CO2 and decreasing aragonite saturation state (Ωar) lead to decreased coral calcification rates and increased dissolution rates of carbonate substrates, the predicted point in time at which an individual reef ecosystem will shift from net accretion to net dissolution varies across studies and ecosystems (Silverman et al. 2007; Ohde and van Woesik 1999; Langdon et al. 2000, 2003; Andersson et al. 2009; Shamberger et al. 2011; Shaw et al. 2012; Andersson et al. 2014). While the global distribution of coral reefs is governed by light availability, temperature, nutrients and Ωar (Kleypas et al. 1999), at the local scale, the conditions that determine coral reef calcification, distribution and community composition are much more complicated. Coral reefs experience high rates of metabolic activity and calcification is influenced by hydrodynamics (e.g., circulation), geomorphology, light, temperature, nutrients and benthic composition (Grigg 1982; Bates et al. 2001; Suzuki and Kawahata 2003; Anthony et al. 2011; Albright et al. 2013; Falter et al. 2013). Furthermore, the complex coupling between physical, chemical and biological dynamics can drive changes in the CO2–carbonic acid system over a diel cycle that are greater than the magnitude of change predicted by the year 2100 (Hofmann et al. 2011; Bopp et al. 2013). Hence, distinguishing between natural variability and anthropogenically driven changes is difficult. Before considering the effects of anthropogenic influence on a system, an understanding of the natural environmental and biological processes that drive the spatial distribution and temporal variability of the inorganic carbon system in an ecosystem must be obtained.
Observing the natural variability of the CO2–carbonic acid system in the French Frigate Shoal (FFS) pristine coral reef ecosystem of the Papahānaumokuākea Marine National Monument (PMNM) provides an opportunity to gain insight into marine inorganic carbon dynamics of an ecosystem minimally impacted by direct human activities. The PMNM is characterized by high coral and fish endemism and exhibits a unique predator-dominated ecosystem structure observed in few places around the world (Friedlander and DeMartini 2002; Maragos et al. 2004; Kane et al. 2013). However, in recent years, indirect effects of human activities have been identified as major threats to PMNM reef ecosystems (Selkoe et al. 2008), including climate change and OA.
Few studies describing the distribution and variability of marine inorganic carbon in PMNM have been published. Sabine and Mackenzie (1995) examined the water column surrounding select atolls within the monument. Their results demonstrated an alkalinity surplus of roughly 10 µmol kg−1 from 0 to 1000 m depth, relative to alkalinity values expected for subtropical waters within the central North Pacific Subtropical Gyre (NPSG) (Millero et al. 1998; Dore et al. 2009; Takahashi et al. 2014). The observed alkalinity surplus (i.e., “the alkalinity halo”) was interpreted to be a consequence of the dissolution of soluble mainly biogenic magnesian calcite particles transitioning offshore from the atoll reefs and lagoons along the island slope. Thompson et al. (2014) analyzed a suite of water samples collected during 2009 and 2010 in waters surrounding the PMNM reefs in order to quantify the extent and magnitude of the alkalinity halo. In contrast, Thompson et al. (2014) found no evidence of an alkalinity halo and concluded that the halo was either an analytical artifact or a temporally variable feature. Kealoha et al. (2015) subsequently examined surface seawater pH and fugacity of carbon dioxide (fCO2) throughout the archipelago using continuous underway sampling. These data showed decreases in surface seawater pH and increases in fCO2 around atolls in PMNM waters and were interpreted as evidence for the impact of coral reef inorganic and organic metabolism on the carbon chemistry of surface seawaters in and around these atolls. In order to gain a more thorough understanding of the impact of reef metabolism on the surface seawaters surrounding the archipelago, we present pH and fCO2 measurements collected around French Frigate Shoals atoll, located in the Northwestern Hawaiian Island (NWHI) chain, via an underway-sampling system at high temporal and spatial resolution.
2 Environmental Setting
An evaluation of the CO2–carbonic acid system in PMNM coastal waters was based on measurements collected at FFS (23°46′N, 166°12′W), a high-latitude reef located at the southern end of the NWHI archipelago. FFS is the largest atoll in the island chain with over 930 km2 of coral reef habitat (Friedlander et al. 2009). The open atoll has a crescent-shaped reef structure on the northwest of the submerged island that feeds into a large lagoon with a mean depth of 42 m. Benthic habitat composition within the lagoon is classified mostly as sand and rubble, with patches of reef and coralline algae. On the eastern boundary, there are numerous low, sandy inlets and a well-developed reef. Winds are predominantly northeasterly trades, which drive open ocean water over the windward reefs and into the lagoon. The western boundary is relatively open to the surrounding ocean and consists of patch reefs. Beyond the lagoon on the leeward side, the bathymetric slope increases rapidly with distance from the atoll. FFS contains the highest coral species richness in PMNM, which reflects optimal conditions for coral growth including low wave exposure, temperatures that range from 23 °C in the winter to 27 °C in the summer, and a protected lagoon environment (Friedlander et al. 2009).
3 Methodology
Continuous measurements of atmospheric and surface seawater CO2 were collected with a General Oceanics Model 8050 Underway pCO2 system with a LI-COR, Inc. 7000 Infrared Gas (CO2/H2O) Analyzer (IRGA) aboard the NOAA Ship Hi`ialakai according to the methods and quality control procedures described in Pierrot et al. (2009). The IRGA is calibrated using four CO2 gas standards (range = 200–450 ppm) in order to verify an accuracy and precision of within ±2 ppm. Sea surface temperature (SST) was determined using a SBE (Sea-Bird Electronic) 38 located at the intake port, 5 m below the sea surface. Sea surface salinity was measured by a SBE 21, and oxygen was measured using an Aanderaa Optode 4330F, corrected for the influences of temperature and salinity. Both the salinity and oxygen sensors were located in the ship’s wet laboratory. In order to calculate CO2 fugacity, temperature and pressure of the equilibrator, water vapor pressure and atmospheric pressure were obtained. Meteorological data were obtained from the National Oceanographic Data Center (NODC) (http://www.nodc.noaa.gov/access/ Accession number: 0104266). Although surface currents were measured with the ship’s Acoustic Doppler Current Profiler (ADCP), the ADCP was programmed to collect data only when the ship was in greater than 50 m of water. Hence, many of the measurements we have within the lagoon, which has a water depth primarily of less than 50 m, do not have coinciding surface current measurements.
Underway surface seawater pH was computed using the voltage output of a DuraFET III (Honeywell, Inc) electrode and an average calibration constant (i.e., standard electrode potential) based on the spectrophotometric pH of 17 discrete samples corrected to in situ temperature. Experimental results indicate the DuraFET can operate with a short-term precision of ±0.0005 over several hours and a stability of ±0.005 over weeks to months (Martz et al. 2010; Bresnahan et al. 2014). Calibration samples were collected into borosilicate glass bottles, poisoned with 0.02% volume of saturated mercuric chloride and sealed with Apiezon grease (Dickson et al. 2007). pH samples were analyzed in the laboratory as soon as the bottle was opened using a Lambda EZ210 spectrophotometer equipped with a temperature-controlled sample chamber (Clayton and Byrne 1993) and purified m-cresol purple dye (Liu et al. 2011) at 25 °C. All pH data are reported on the total scale.
Since certified reference materials (CRMs) for pH were not available, pH was calculated from the total alkalinity (TA) and dissolved inorganic carbon (DIC) values of CRMs provided by Dickson (Scripps Institution of Oceanography) using CO2SYS (Pierrot et al. 2006) and the equilibrium constants of Mehrbach et al. (1973) refit on the total pH scale, which includes the dissociation of bisulfate, by Dickson and Millero (1987). Based on the offset between the calculated pH from CRMs and our discrete samples, the mean difference was 0.006 ± 0.003 (mean ± SD). The reproducibility of the discrete pH samples, based on 33 sets of duplicates, was 0.0002 ± 0.007. In order to verify the precision of the pH electrode, the differences between each electrode measurement taken every 2 min over a 12-h period were calculated. These pairs were assumed to be representative of duplicates since the measurements were taken in the open ocean while transiting between islands. The mean difference for the pH electrode duplicates was ±0.0006, which is consistent with the findings of Martz et al. (2010).
In order to assess the impact of inorganic metabolism (calcification/dissolution) on waters surrounding FFS, TA and DIC were calculated from fCO2 and pH. However, there are potentially large errors associated with this calculation (Dickson and Riley 1978; Millero 2007; Cullison Gray et al. 2011). A precision of ±2 µatm for fCO2 and ±0.0006 for the pH sensor generates a DIC reproducibility of ±12 µmol kg−1, with similar results for TA. The uncertainty (e.g., accuracy) in the fCO2 and spectrophotometric pH measurements (±0.006) used to calibrate the electrode may produce a ±30–50 µmol kg−1 uncertainty in TA and DIC. Hence, the error in the accuracy of pH and fCO2 may shift the entire set of TA and DIC values higher or lower relative to the true values. However, the precision of the measurements is reasonable and therefore produces reliable trends. The uncertainties in our pH and fCO2 measurements are propagated through the calculation for TA and DIC and the error is therefore reflected in the TA: DIC ratio. Despite the potential error in the calculation, we then used the ratio between TA, normalized to a salinity of 35 (nTA), and salinity-normalized DIC (nDIC) to evaluate the relationship between inorganic and organic coral reef metabolism.
These measurements were collected over 1000 km2 of ocean surface that include waters within the lagoon and the open ocean, while the ship was navigated in and around FFS in support of a number of research objectives. Given the location and large area over which these measurements were obtained, they are not strictly representative of metabolic processes that occur over the reef. Rather, they encompass modifications in seawater inorganic carbon chemistry induced by physical (e.g., mixing, currents and tides) and biogeochemical processes that occurred in upstream habitats and throughout the ecosystem. These observations are neither strictly Eulerian or Lagrangian in nature, which makes it difficult to determine the origin of water parcels and the transit times to a particular sampling location. Furthermore, because the ship steamed in open ocean waters at night, these measurements probably do not encompass the full range of diel patterns produced by metabolic processes over the reef and lagoon. In order to interpret these observations, we have examined our data in terms of change through time and change over space. Although disentangling spatially driven trends from temporally driven trends in this dataset is difficult, we provide the first description of the surface seawater CO2–carbonic acid system dynamics in and around FFS.
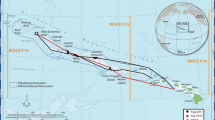
Approximately 4 days (7/7–7/10) were spent at FFS during July 2012 (Fig. 1). All surface seawater measurements collected within 40 km of the 50-m depth contour were included in this analysis. Distance (km) from the 50-m depth contour surrounding the atoll was used as an indicator of the ship’s position relative to the reef ecosystem. Positive values were assigned to distances inside the 50-m depth contour, and negative values were assigned to distances outside the 50-m depth contour. The ship typically remained inside the 50-m depth contour from 6:00 to 18:00 and outside the 50-m depth contour from 18:00 to 6:00. On days two and three, the ship sampled within the lagoon near the center of the atoll, whereas day four of operations was conducted on the northwest side of the atoll.
The ship arrived at FFS the night of July 7th. Underway sample collection on July 8th and 9th was conducted in the lee of the forereef, whereas sampling on July 10th was conducted on the northwest corner of the atoll (Fig. 1). The northwest corner of FFS is exposed to open ocean water moving past the atoll, possibly confounding the signal contributed by coral reef metabolism. The data collected on July 7th before the ship arrived at FFS and the data collected over the northwest corner of the atoll on July 10th therefore are not included in the discussion section.
4 Results
All physical and biogeochemical parameters including temperature, salinity, oxygen, pH and fCO2, displayed greater variability within the 50-m depth contour. Temperature and salinity ranged from ~25.6 to 26.2 °C and ~ 35.8–36.0, respectively, with higher values generally observed inside the atoll (i.e., inside the 50-m contour) (Fig. 2). Oxygen (range = 6.11–7.93 mg L−1) was lower in the mornings when the ship entered the atoll and increased throughout day. Outside the atoll (i.e., outside the 50-m contour) at night, oxygen was higher and less variable. CO2 fugacity varied between 380 and 440 µatm and was generally higher inside the lagoon, and lower and less variable outside the atoll (Fig. 3). pH ranged between 7.99–8.05 and was consistently lower inside the atoll. Measurements of low oxygen and pH, and high fCO2 inside the atoll in the mornings are consistent with increased respiration and remineralization of organic matter, and likely a residual product of nighttime processes (see Sect. 5). In order to assess quantitatively the variability of the pH and fCO2 measurements, we calculated the coefficient of variation (CV) for these variables both inside and outside of the atoll. As expected, pH and fCO2 show 60 and 40% more variability, respectively, inside the atoll relative to the open ocean. Furthermore, if we hold TA and DIC constant and introduce the variability in temperature (0.6 °C) and salinity (0.2 ppt), fCO2 changes by approximately 10 µatm and pH by ~0.01 units. Hence, 80% of the variability in fCO2 and 98% of the variability in pH can be attributed to metabolic and mixing processes.
Temperature (a), salinity (b) and oxygen saturation (c) in red over 4 days at FFS during July 2012. The secondary y-axis indicates the measurement location in relation to the 50-m depth contour where distances greater than 0 km are inside the 50-m depth contour (i.e., inside the atoll) and distances less than 0 km are outside the 50-m depth contour (i.e., outside the atoll). The track of the ship in relation to the 50-m contour is show in black
Surface water fCO2 (a) and pH (b) in red over 4 days at FFS during July 2012. The secondary y-axis indicates the measurement location in relation to the 50-m depth contour where distances greater than 0 km are inside the 50-m depth contour (i.e., inside the atoll) and distances less than 0 km are outside the 50-m depth contour (i.e., outside the atoll). The track of the ship in relation to the 50-m contour is show in black
5 Discussion
Under the narrow ranges of temperature and salinity (Fig. 2) that we see during this study, the variability in fCO2 and pH inside the atoll (Fig. 3) is a clear indication that metabolic processes are altering the carbonate chemistry of the surface seawater. However, fCO2 and pH are influenced by both inorganic (calcification/dissolution) and organic (photosynthesis/respiration) metabolism as well as air-sea exchange, whereas alkalinity primarily reflects calcification and carbonate dissolution. In the following sections, we use calculated TA and DIC to illustrate spatial trends in marine inorganic carbon chemistry, as well as characterize the relative impact of inorganic and organic metabolism on this chemistry, in waters at FFS.
5.1 Spatial scale impact of reef inorganic metabolism
There exists a remarkably conspicuous oscillation in nTA (Fig. 4) of waters within FFS lagoon and outside, encompassing an area of 300 km2, indicating that metabolic and physical processes influence the chemistry of surface seawater at considerable distances from the atoll. These measurements show that nTA increases from 6:00 to 18:00 and decreases from 18:00 to 6:00 (Fig. 4). It is well established that for carbonate ecosystems, calcification decreases TA during the day and carbonate dissolution increases TA at night (e.g., Shamberger et al. 2011; Shaw et al. 2012; Albright et al. 2013), and hence this oscillation clearly does not represent the in situ diel calcification/dissolution signal generated over the reef flat. These underway data were collected roughly 8–10 km downwind from the forereef during the day and 20 km downwind at night. Therefore, the chemical signals being measured result from a combination of the biogeochemical and physical processes occurring at FFS. These processes may include advection of water containing the signature of upstream reef metabolism, dissolution of carbonate minerals within the lagoon, which is driven by in situ respiration and remineralization of organic matter, and mixing induced by tides and currents. Given the complicated nature of this dataset, we are not able to separate the temporal and spatial components within this trend. Rather, we wish to emphasize the magnitude of the nTA oscillation and the large extent of area (hundreds of square kilometers of reef, lagoon and open ocean) that experiences both high (+120 µmol kg−1) and low (−150 µmol kg−1) alkalinity relative to the stable nTA values found in the NPSG (Millero et al. 1998; Key et al. 2004).
Five-point mean average of nTA over 2 days at FFS during July 2012. nTA is calculated from pCO2 and pH measured every 2 min in the surface waters within 40 km of the 50-m depth contour. The color of each individual measurement represents the location of that sample with respect to distance from the 50-m depth contour. Negative distances are outside the 50-m depth contour and positive distances are inside the 50-m depth contour
Clear island-to-open ocean gradients were observed in nTA as the research vessel moved closer to and farther from the FFS reef flat over two consecutive days. Alkalinity was depleted nearshore by approximately 150 µmol kg−1, increased to an excess of 120 μmol kg−1 near the 50-m depth contour, then remained relatively constant 10–20 km outside the 50-m depth contour (Fig. 5). Since nTA values observed at maximum distances from the island are roughly equal to the 2002–2012 average surface TA, normalized to a salinity of 35 ppt, at Station ALOHA of ~2305 ± 4 µmol kg−1 (http://hahana.soest.hawaii.edu/hot/hot-dogs/), we are confident that our calculated nTA is representative of the actual nTA within the estimated accuracy of 30–50 µmol kg−1.
Calculated nTA as a function of distance at FFS during 2 days in July 2012. Positive distances are inside the 50-m depth contour and negative distances are outside the 50-m depth contour. The circles (o) represent data collected on 07/08/12 and the crosses (+) represent data collected on 07/09/12. The colors of the symbols represent time of day the measurement was collected
On both days, elevated nTA (Fig. 5) and elevated fCO2 values (Fig. 3) were observed in surface waters over the island slope. Interestingly, this feature was observed in the evenings (blue symbols in Fig. 5) when transiting into deep water, but was absent in the mornings when transiting into the atoll. There are several processes that may produce this alkalinity surplus, which may be the same feature previously referred to as the “alkalinity halo” (Sabine and Mackenzie 1995; Thompson et al. 2014). As previously discussed, in situ respiration and remineralization of organic matter within the lagoon may drive dissolution, or water parcels containing the signature of nighttime metabolic processes (i.e., respiration and dissolution) over the reef may have been advected to this location. Reef and lagoon waters are subsequently influenced by tides, horizontal mixing, and potentially, topographically induced vertical mixing, which has been observed along the Hawaiian Ridge (Garrett 2003; Martin and Rudnick 2007). Hence, through these physical processes, the dissolution signal may have been mixed out by the morning. While we are unable to pinpoint the specific physical and biogeochemical process(es) that produce the dissolution signal, our measurements suggest that this feature is temporally variable and during the time of our field work, occurred in the evenings, and along the island slope.
5.2 TA: DIC Relationship
The coupling between coral reef organic and inorganic metabolism is well established. Photosynthesis during the day consumes DIC and increases Ωar, which promotes calcification that depletes TA (Suzuki et al. 1995; Ohde and van Woesik 1999; Bates et al. 2010; Shamberger et al. 2011; Lantz et al. 2013). At night, respiration produces CO2, decreases Ωar, and calcification often slows, with some benthic communities experiencing net dissolution (Yates and Halley 2003, 2006; Andersson and Gledhill 2013; Eyre et al. 2014). The impact of these processes on the variability in the parameters of the CO2–carbonic acid system depends on a number of environmental factors including geographic location, seasonal and daily trends in temperature and light availability, hydrodynamic forcing and nutrients (Suzuki et al. 1995; Andersson et al. 2005; Bates et al. 2010; Drupp et al. 2011, 2013; Falter et al. 2012; Zhang et al. 2012; Yeakel et al. 2015). Furthermore, community composition upstream of a particular reef environment can alter seawater chemistry and complicate the interpretation of research results in downstream habitats (Anthony et al. 2011; Kleypas et al. 2011).
The ΔTA: ΔDIC relationship reflects the balance between net ecosystem calcification (NEC) and net primary production (NPP) (Gattuso et al. 1996; Watanabe et al. 2006; Andersson and Gledhill 2013; Lantz et al. 2013; Mackenzie and Andersson 2013). If TA and DIC decrease at a rate greater than one, calcification exceeds primary production and if TA and DIC decrease at a rate less than one, primary production exceeds calcification. Although this relationship is shown to be independent of external environmental forcings such as solar irradiance and residence time of water, it varies in response to changes in ecosystem function. For example, OA, coral bleaching, and eutrophication may induce a shift from coral to algal-dominated reefs, processes that will decrease calcification, increase primary production, and decrease the slope of TA versus DIC. Therefore, the ΔTA: ΔDIC ratio has been proposed as a simple tool for monitoring coral reef health, particularly in response to OA (Andersson and Gledhill 2013; Lantz et al. 2013). The rate of change in TA relative to DIC over time (also called the slope or reaction pathway) is typically slightly less than or near one, where photosynthesis and respiration are the prevalent controls on variations in Ωar (Massaro et al. 2012; Andersson and Gledhill 2013).
The normalized ΔTA: ΔDIC ratio measured inside the 50-m depth contour over 2 days at FFS exhibits a dominant slope of ~1.04 (Fig. 6). This is close to the TA: DIC ratio observed at other coral reefs (Massaro et al. 2012; Andersson and Gledhill 2013). The chemical reactions that lead to the 1:1 slope are:
where inorganic and organic metabolism are generally balanced, producing little variations in Ωar. In the morning (~6:00), as the ship approaches its closest position to the reef (8 km inside the 50-m depth contour), nTA and nDIC are depleted relative to the open ocean (Fig. 6a). pH decreases (Fig. 3), fCO2 increases (Figs. 3, 6b), there is an additional input of DIC (Fig. 6) and oxygen decreases (Fig. 6c). These patterns persist until at least mid-day and are consistent with heterotrophy. Perhaps these patterns are remnants of nighttime respiration that occurred over the reef. Since oxygen remains low for the first half of the day, there may be an additional remineralization of organic matter within the lagoon system. Most lagoons are slightly net heterotrophic (Kinsey 1979), although Atkinson and Grigg (1984) measured a P/R (photosynthesis/respiration) ratio of 1.06 in the summer at FFS. However, Atkinson and Grigg (1984) do postulate their estimated P/R may have been too high. Clearly, there is spatial heterogeneity and mixing of different water masses, which is evident in the patchiness and fluctuations in the chemical parameters we have measured. Mixing is likely induced by wind-driven currents, the tidal cycle, or some combination of both.
Property–property plots of nDIC and nTA calculated from the pH-pCO2 pair. These data were collected during July 2012 and are all inside the 50-m depth contour. a nTA and nDIC where colors represent distance and contour lines represent Ωar. A distance of 0 km represents data collected on the 50-m depth contour and greater distances represent measurements collected further inside the atoll. b nTA and nDIC where colors represent sea–air CO2 and contour lines represent fCO2 and c nTA and nDIC where colors represent oxygen concentration
6 Conclusion
In spite of the spatial and temporal complexities associated with this dataset, a number of important features and trends emerged. Clearly, the use of an underway system with continuous sampling for fCO2 coupled with the highly precise Honeywell DuraFET electrode provides data useful for observing CO2–carbonic acid system dynamics of coral reef ecosystems over large spatial scales. Our fCO2 and pH measurements reflect the impact of reef metabolism on the surface seawater inorganic carbon system up to 10–20 km outside the 50-m depth contour and demonstrate that this metabolism can either elevate or depress alkalinity values over large areas of the ocean adjacent to coral reefs and probably other carbonate systems. This observation supports the finding of Sabine and Mackenzie (1995) for an alkalinity halo in and around NWHI atolls and the hypothesis of Thompson et al. (2014) that the differences in the magnitude of the alkalinity halo are a result of temporal variability, perhaps driven by hydrodynamic forcings (e.g., circulation and mixing), temporal trends in reef metabolism and/or net heterotrophy within the lagoon system.
References
Albright R, Langdon C, Anthony KRN (2013) Dynamics of seawater carbonate chemistry, production, and calcification of a coral reef flat, central Great Barrier Reef. Biogeosciences 10(10):6747–6758
Andersson AJ, Gledhill D (2013) Ocean acidification and coral reefs: effects on breakdown, dissolution, and net ecosystem calcification. Annu Rev Mar Sci 5:1.1–1.28
Andersson AJ, Mackenzie FT, Lerman A (2005) Coastal ocean and carbonate systems in the high CO2 world of the Anthropocene. Am J Sci 305:875–918
Andersson AJ, Kuffner IB, Mackenzie FT, Jokiel PL, Rodgers KS, Tan A (2009) Net loss of CaCO3 from a subtropical calcifying community due to seawater acidification: mesocosm-scale experimental evidence. Biogeosciences 6:1811–1823
Andersson AJ, Yeakel KL, Bates NR, de Putron SJ (2014) Partial offsets in ocean acidification from changing coral reef biogeochemistry. Nat Clim Change 4:56–61
Anthony K, Kleypas J, Gattuso JP (2011) Coral reefs modify their seawater carbon chemistry—implications for impacts of ocean acidification. Glob Change Biol 17(12):3655–3666
Atkinson MJ, Grigg RW (1984) Model of a coral reef ecosystem II. Gross and net benthic primary production at French Frigate Shoals, Hawaii. Coral Reefs 3:13–22
Bates NR, Samuels L, Merlivat L (2001) Biogeochemical and physical factors influencing seawater fCO2 and air–sea CO2 exchange on the Bermuda coral reef. Limnol Oceanogr 46(4):833–846
Bates NR, Amat A, Andersson AJ (2010) Feedbacks and responses of coral calcification on the Bermuda reef system to seasonal changes in biological processes and ocean acidification. Biogeosciences 7:2509–2530
Bopp L, Resplandy L, Orr JC, Doney SC, Dunne JP, Gehlen M, Halloran P, Heinze C, Ilyina T, Seferian R, Tjiputra J, Vichi M (2013) Multipe stressors of ocean ecosystems in the 21st century: projection with the CMIP5 models. Biogeosciences 10:6225–6245
Bresnahan PJ, Martz TR, Takeshita Y, Johnson KS, LaShomb M (2014) Best practices for autonomous measurement of seawater pH with the Honeywell Durafet. Methods Oceanogr 9:44–60
Caldeira K, Wickett ME (2003) Oceanography: anthropogenic carbon and ocean pH. Nature 425(6956):365
Cesar H, Burke L, Pet-Soede L (2003) The economics of worldwide coral reef degradation. Cesar Environmental Economics Consulting, the Netherlands
Clayton T, Byrne R (1993) Spectrophotometric seawater pH measurements: total hydrogen ion concentration scale calibration of m-cresol purple and at-sea results. Deep Sea Res I 40(10):2115–2129
Cullison Gray SE, DeGrandpre MD, Moore TS, Martz TR, Friederich GE, Johnson KS (2011) Applications of in situ pH measurements for inorganic carbon calculations. Mar Chem 125(1):82–90
Dickson AG, Millero FJ (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res A Oceanogr Res Pap 34(10):1733–1743
Dickson AG, Riley JP (1978) The effect of analytical error on the evaluation of the components of the aquatic carbon-dioxide system. Mar Chem 6(1):77–85
Dickson A, Sabine C, Christian J (eds) (2007) Guide to best practices for ocean CO2 measurements. PICES Special Publication 3
Donner SD, Potere D (2007) The inequity of the global threat to coral reefs. Bioscience 57(3):214–215
Dore JE, Lukas R, Sadler D, Church MJ, Karl DM (2009) Physical and biogeochemical modulation of ocean acidification in the central North Pacific. Proc Natl Acad Sci USA 106(20):12235–12240
Drupp PS, De Carlo EH, Mackenzie FT, Bienfang P, Sabine CL (2011) Nutrient inputs, phytoplankton response, and CO2 variations in a semi-enclosed subtropical embayment, Kaneohe Bay, Hawaii. Aquat Geochem 17:473–498
Drupp PS, De Carlo EH, Mackenzie FT, Sabine CL, Feely RA, Shamberger KE (2013) Comparison of CO2 dynamics and air–sea gas exchange in differing tropical reef environments. Aquat Geochem 19:371–397
Eyre BD, Andersson AJ, Cyronak T (2014) Benthic coral reef calcium carbonate dissolution in an acidifying ocean. Nat Clim Chang 4(11):969–976
Falter JL, Lowe RJ, Atkinson MJ, Cuet P (2012) Seasonal coupling and de-coupling of net calcification rates from coral reef metabolism and carbonate chemistry at Ningaloo Reef, Western Australia. J Geophys Res Oceans. doi:10.1029/2011JC007268
Falter JL, Lowe RJ, Zhang Z, McCulloch M (2013) Physical and biological controls on the carbonate chemistry of coral reef waters: effects of metabolism, wave forcing, sea level, and geomorphology. PLoS ONE 8(1):e53303
Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry CJ, Millero FJ (2004) Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305(5682):362–366
Friedlander AM, DeMartini EE (2002) Contrasts in density, size, and biomass of reef fishes between the northwestern and the main Hawaiian Islands: the effects of fishing down apex predators. Mar Ecol Prog Ser 230:253–264
Friedlander A, Keller K, Wedding L, Clarke A, Monaco M (2009) A marine biogeographic assessment of the Northwestern Hawaiian Islands. NOAA Technical Memorandum NOS NCCOS 84. Prepared by NCCOS’s Biogeography Branch in cooperation with the Office of National Marine Sanctuaries Papahanaumokuakea
Garrett C (2003) Internal tides and ocean mixing. Science 301(5641):1858–1859
Gattuso J-P, Pichon M, Delesalle B, Canon C, Frankignoulle M (1996) Carbon fluxes in coral reefs. I. Lagrangian measurement of community metabolism and resulting air–sea CO2 disequilibrium. Mar Ecol Prog Ser 145:109–121
Grigg RW (1982) Darwin point: a threshold for atoll formation. Coral Reef 1:29–34
Hofmann GE, Smith JE, Johnson KS, Send U, Levin LA, Micheli F, Paytan A, Price NN, Peterson B, Takeshita Y, Matson PG, Crook ED, Kroeker KJ, Gambi MC, Rivest EV, Frieder CA, Yu PC, Martz TR (2011) High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6(12):e28983
Kane C, Kosaki R, Wagner D (2013) High levels of mesophotic reef fish endemism in the Northwestern Hawaiian Islands. Bull Mar Sci 90(2):693–703
Kealoha AK, Kahng SE, Mackenzie FT, Alin SR, Kosaki RK, Brainard RE, Winn CD (2015) Latitudinal trends and drivers in the CO2–carbonic acid system of Papahānaumokuākea Marine National Monument. Aquat Geochem. doi:10.1007/s10498-015-9273-z
Key RM, Kozyr A, Sabine CL, Lee K, Wanninkhof R, Bullister JL, Feely RA, Millero FJ, Mordy C, Peng T-H (2004) A global ocean carbon climatology: results from Global Data Analysis Project (GLODAP). Glob Biogeochem Cycles 18(4):GB4031
Kinsey DW (1979) Carbon turnover and accumulation by coral reefs. PhD dissertation, University of Hawaii, Honolulu
Kleypas JA, McManus JW, Meñez LAB (1999) Environmental limits to coral reef development: Where do we draw the line? Am Zool 39(1):146–159
Kleypas JA, Anthony K, Gattuso JP (2011) Coral reefs modify their seawater carbon chemistry–case study from a barrier reef (Moorea, French Polynesia). Glob Change Biol 17(12):3667–3678
Langdon C, Takahashi T, Sweeney C, Chipman D, Goddard J, Marubini F, Aceves H, Barnett H, Atkinson MJ (2000) Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Glob Biogeochem Cycles 14(2):639–654
Langdon C, Broeker WS, Hammond DE, Glenn E, Fitzsimmons K, Nelson SG, Peng TH, Hajdas I, Bonani G (2003) Effect of elevated CO2 on the community metabolism of an experimental coral reef. Global Biogeochem Cycles 17:1011
Lantz CA, Atkinson MJ, Winn CW, Kahng SE (2013) Dissolved inorganic carbon and total alkalinity of a Hawaiian fringing reef: chemical techniques for monitoring the effects of ocean acidification on coral reefs. Coral Reefs 33(1):105–115
Liu X, Patsavas MC, Byrne RH (2011) Purification and characterization of meta-cresol purple for spectrophotometric seawater pH measurements. Environ Sci Technol 45(11):4862–4868
Mackenzie FT, Andersson AJ (2013) The marine carbon system and ocean acidification during Phanerozoic time. Geochem Perspect 2:1–227
Maragos JE, Potts DC, Aeby GS, Gulko D, Kenyon J, Siciliano D, VanRavenswaay D (2004) 2000-2002 rapid ecological assessment of corals (Anthozoa) on shallow reefs of the Northwestern Hawaiian Islands. Part 1: species and distribution. Pac Sci 58(2):211–230
Martin JP, Rudnick DL (2007) Inferences and observations of turbulent dissipation and mixing in the upper ocean at the Hawaiian Ridge. J Phys Oceanogr 37(3):476–494
Martz TR, Connery JG, Johnson KS (2010) Testing the Honeywell Durafet for seawater pH applications. Limnol Oceanogr: Methods. 8:172–184
Massaro RFS, De Carlo EH, Drupp PS, Mackenzie FT, Jones SJ, Shamberger KE, Sabine CL, Feely RA (2012) Multiple factors driving variability of CO2 exchange between the ocean and atmosphere in a tropical coral reef environment. Aquat Geochem 18:357–386
Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RM (1973) Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr 18(6):897–907
Millero FJ (2007) The marine inorganic carbon cycle. Chem Rev 107(2):308–341
Millero FJ, Lee K, Roche M (1998) Distribution of alkalinity in the surface waters of the major oceans. Mar Chem 60:111–129
Ohde S, Van Woesik R (1999) Carbon dioxide flux and metabolic processes of a coral reef, Okinawa. Bull Mar Sci 65(2):559–576
Pierrot D, Lewis E, Wallace DWR (2006) MS Excel program developed for CO2 system calculations. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, Oak Ridge
Pierrot D, Neill C, Sullivan K, Castle R, Wanninkhof R, Luger H, Johannessen T, Olsen A, Feely R, Cosca C (2009) Recommendations for autonomous underway pCO2 measuring system and data-reduction routines. Deep Sea Res II 56:512–522
Sabine CL, Mackenzie FT (1995) Bank-derived carbonate sediment transport and dissolution in the Hawaiian Archipelago. Aquat Geochem 1(2):189–230
Selkoe KA, Halpern BS, Toonen R (2008) Evaluating anthropogenic threats to the Northwestern Hawaiian Islands. Aquat Conserv Mar Freshw Ecosyst 18(7):1149–1165
Shamberger KEF, Feely RA, Sabine CL, Atkinson MJ, De Carlo EH, Mackenzie FT, Drupp PS, Butterfield DA (2011) Calcification and organic production on a Hawaiian coral reef. Mar Chem 127:64–75
Shaw EC, McNeil BI, Tilbrook B (2012) Impacts of ocean acidification in naturally variable coral reef flat ecosystems. J Geophys Res 117:C03038
Silverman J, Lazar B, Erez J (2007) Effect of aragonite saturation, temperature, and nutrients on the community calcification rate of a coral reef. J Geophys Res 112:C05004
Smith SV (1978) Coral-reef area and the contributions of reefs to processes and resources of the world’s oceans. Nature 273:225–226
Smith SV, Mackenzie FT (2016) The role of CaCO3 reactions in the contemporary oceanic CO2 cycle. Aquat Geochem 22(2):153–175
Suzuki A, Kawahata H (2003) Carbon budget of coral reef systems: an overview of observations in fringing reefs, barrier reefs and atolls in the Indo-Pacific regions. Tellus 55B:428–444
Suzuki A, Nakamori T, Kayanne H (1995) The mechanism of production enhancement in coral reef carbonate systems: model and empirical results. Sediment Geol 99(3):259–280
Takahashi T, Sutherland SC, Chipman DW, Goddard JG, Ho C, Newberger T, Sweeney C, Munro DR (2014) Climatological distributions of pH, pCO2, total CO2, alkalinity, and CaCO3 saturation in the global surface ocean, and temporal changes at selected locations. Mar Chem 164:95–125
Thompson RW, Dickson AG, Kahng SE, Winn CD (2014) Nearshore Carbonate dissolution in the Hawaiian Archipelago? Aquat Geochem. doi:10.1007/s10498-014-9230-2
Van Hooidonk R, Maynard JA, Manzello D, Planes S (2014) Opposite latitudinal gradients in projected ocean acidification and bleaching impacts on coral reefs. Glob Change Biol 20:103–112
Watanabe A, Kayanne H, Hata H, Kudo S, Nozaki K, Kato K, Negishi A, Ikeda Y, Yamano H (2006) Analysis of the seawater CO2 system in the barrier reef-lagoon system of Palau using total alkalinity-dissolved inorganic carbon diagrams. Limnol Oceanogr 51(4):1614–1628
Yates KK, Halley RB (2003) Measuring coral reef community metabolism using new benthic chamber technology. Coral Reefs 22(3):247–255
Yates KK, Halley RB (2006) Diurnal variation in rates of calcification and carbonate sediment dissolution in Florida Bay. Estuar Coast 29(1):24–39
Yeakel KL, Andersson AJ, Bates NR, Noyes TJ, Collins A, Garley R (2015) Shifts in coral reef biogeochemistry and resulting acidification linked to offshore productivity. Proc Natl Acad Sci USA 112(47):14512–14517
Zhang Z, Falter J, Lowe R, Ivey G (2012) The combined influence of hydrodynamic forcing and calcification on the spatial distribution of alkalinity in a coral reef system. J Geophys Res Oceans. doi:10.1029/2011JC007603
Acknowledgements
The authors would like to thank Cathy Cosca and Geoff Lebon of NOAA Pacific Marine Environmental Laboratory, NOAA's Coral Reef Ecosystem Program, as well as the NOAA Hi’ialakai ship crew. This work would not have been possible without their ongoing support through data management, mechanical expertise and science support. The authors would also like to thank the two reviewers who provided detailed comments and recommendations and greatly helped to improve this manuscript. This work was funded by NOAA’s Office of National Marine Sanctuaries, Papahānaumokuākea Marine National Monument. This is PMEL contribution number 4600.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kealoha, A.K., Mackenzie, F.T., Kahng, S.E. et al. Spatiotemporal Assessment of CO2–Carbonic Acid System Dynamics in a Pristine Coral Reef Ecosystem, French Frigate Shoals, Northwestern Hawaiian Islands. Aquat Geochem 23, 75–88 (2017). https://doi.org/10.1007/s10498-017-9310-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-017-9310-1