Abstract
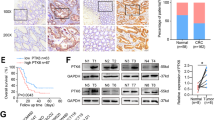
KRAS mutations are one of the most prevalent genetic alterations in colorectal cancer (CRC). Although directly targeting KRAS still is a challenge in anti-cancer therapies, alternatively inhibiting KRAS related signaling pathways has been approached effectively. Here we firstly reported that MAP kinase, transforming growth factor-β-activated kinase 1 (TAK1), commonly expressed in CRC cell lines and significantly associated with KRAS mutation status. Inhibition of TAK1 by the small molecular inhibitor NG25 could inhibit CRC cells proliferation in vitro and in vivo, especially in KRAS-mutant cells. NG25 induced caspase-dependent apoptosis in KRAS-mutant cells and in orthotopic CRC mouse models by regulating the B-cell lymphoma-2 (Bcl-2) family and the inhibitor of apoptosis protein (IAP) family. Besides inhibiting molecules downstream of MAPK, including ERK, JNK and p38 phosphorylation, NG25 could block NF-κB activation in KRAS-mutant cells. As a target gene of NF-κB, down-regulated XIAP expression may be not only involved in apoptosis induced by NG25, but also reducing the formation of TAK1-XIAP complex that can activate TAK1 downstream signaling pathways, which forms a positive feedback loop to further induce apoptosis in KRAS-mutant CRC cells. Together, these findings indicated that TAK1 is an important kinase for survival of CRCs harboring KRAS mutations, and that NG25 may be a potential therapeutic strategy for KRAS-mutant CRC.







Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386. https://doi.org/10.1002/ijc.29210
Siegel RL, Miller KD, Jemal A (2017) Cancer Statistics, 2017. CA Cancer J Clin 67(1):7–30. https://doi.org/10.3322/caac.21387
De Roock W, De Vriendt V, Normanno N, Ciardiello F, Tejpar S (2011) KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol 12(6):594–603. https://doi.org/10.1016/s1470-2045(10)70209-6
Herreros-Villanueva M, Chen CC, Yuan SS, Liu TC, Er TK (2014) KRAS mutations: analytical considerations. Clin Chim Acta 431:211–220. https://doi.org/10.1016/j.cca.2014.01.049
Whyte DB, Kirschmeier P, Hockenberry TN, Nunez-Oliva I, James L, Catino JJ, Bishop WR, Pai JK (1997) K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem 272(22):14459–14464
Singh A, Sweeney MF, Yu M, Burger A, Greninger P, Benes C, Haber DA, Settleman J (2012) TAK1 inhibition promotes apoptosis in KRAS-dependent colon cancers. Cell 148(4):639–650. https://doi.org/10.1016/j.cell.2011.12.033
Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K (1995) Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science 270(5244):2008–2011
Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K (1999) The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398(6724):252–256. https://doi.org/10.1038/18465
Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ (2001) TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412(6844):346–351. https://doi.org/10.1038/35085597
Chen YR, Tan TH (2000) The c-Jun N-terminal kinase pathway and apoptotic signaling (review). Int J Oncol 16(4):651–662
Mihaly SR, Ninomiya-Tsuji J, Morioka S (2014) TAK1 control of cell death. Cell Death Differ 21(11):1667–1676. https://doi.org/10.1038/cdd.2014.123
Tan L, Nomanbhoy T, Gurbani D, Patricelli M, Hunter J, Geng J, Herhaus L, Zhang J, Pauls E, Ham Y, Choi HG, Xie T, Deng X, Buhrlage SJ, Sim T, Cohen P, Sapkota G, Westover KD, Gray NS (2015) Discovery of type II inhibitors of TGFbeta-activated kinase 1 (TAK1) and mitogen-activated protein kinase kinase kinase kinase 2 (MAP4K2). J Med Chem 58(1):183–196. https://doi.org/10.1021/jm500480k
Wang Z, Zhang H, Shi M, Yu Y, Wang H, Cao WM, Zhao Y, Zhang H (2016) TAK1 inhibitor NG25 enhances doxorubicin-mediated apoptosis in breast cancer cells. Sci Rep 6:32737. https://doi.org/10.1038/srep32737
Hrabe JE, O’Leary BR, Fath MA, Rodman SN, Button AM, Domann FE, Spitz DR, Mezhir JJ (2015) Disruption of thioredoxin metabolism enhances the toxicity of transforming growth factor beta-activated kinase 1 (TAK1) inhibition in KRAS-mutated colon cancer cells. Redox Biol 5:319–327. https://doi.org/10.1016/j.redox.2015.06.004
Khanbolooki S, Nawrocki ST, Arumugam T, Andtbacka R, Pino MS, Kurzrock R, Logsdon CD, Abbruzzese JL, McConkey DJ (2006) Nuclear factor-kappaB maintains TRAIL resistance in human pancreatic cancer cells. Mol Cancer Ther 5(9):2251–2260. https://doi.org/10.1158/1535-7163.mct-06-0075
Du J, Wang Y, Chen D, Ji G, Ma Q, Liao S, Zheng Y, Zhang J, Hou Y (2016) BAY61-3606 potentiates the anti-tumor effects of TRAIL against colon cancer through up-regulating DR4 and down-regulating NF-kappaB. Cancer Lett 383(2):145–153. https://doi.org/10.1016/j.canlet.2016.10.002
Yun SI, Kim HH, Yoon JH, Park WS, Hahn MJ, Kim HC, Chung CH, Kim KK (2015) Ubiquitin specific protease 4 positively regulates the WNT/beta-catenin signaling in colorectal cancer. Mol Oncol 9(9):1834–1851. https://doi.org/10.1016/j.molonc.2015.06.006
He X, Shi W, Wen S, SUN Y, Ling G, Shen k, Peng C, Chen B, Wang J (2015) The establishment and evaluation of orthotopic colorectal cancer model in cecum mesentery triangle Chinese J Oncol 37(6):418–421. https://doi.org/10.3760/cma.j.issn.0253-3766.2015.06.004
Rumble JM, Duckett CS (2008) Diverse functions within the IAP family. J Cell Sci 121(Pt 21):3505–3507. https://doi.org/10.1242/jcs.040303
Chua CWL, Chong DQ, Kanesvaran R, Tai WMD, Tham CK, Tan P, Earnest A, Tan IB (2014) The prognostic impact of KRAS mutation in colorectal cancer patients: a meta-analysis of phase III clinical trials. J Clin Oncol 32(15_suppl):e14515–e14515. https://doi.org/10.1200/jco.2014.32.15_suppl.e14515
Hutchins G, Southward K, Handley K, Magill L, Beaumont C, Stahlschmidt J, Richman S, Chambers P, Seymour M, Kerr D, Gray R, Quirke P (2011) Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol 29(10):1261–1270. https://doi.org/10.1200/JCO.2010.30.1366
Dahabreh IJ, Terasawa T, Castaldi PJ, Trikalinos TA (2011) Systematic review: Anti-epidermal growth factor receptor treatment effect modification by KRAS mutations in advanced colorectal cancer. Ann Intern Med 154(1):37–49. https://doi.org/10.7326/0003-4819-154-1-201101040-00006
Gavrilescu LC, Molnar A, Murray L, Garafalo S, Kehrl JH, Simon AR, Van Etten RA, Kyriakis JM (2012) Retraction for Zhong et al. GCK is essential to systemic inflammation and pattern recognition receptor signaling to JNK and p38. Proc Natl Acad Sci USA 109(13):5134. https://doi.org/10.1073/pnas.1203089109
Wang H, Chen Z, Li Y, Ji Q (2017) NG25, an inhibitor of transforming growth factorbetaactivated kinase 1, ameliorates neuronal apoptosis in neonatal hypoxicischemic rats. Mol Med Rep. https://doi.org/10.3892/mmr.2017.8024
Lewis J, Burstein E, Reffey SB, Bratton SB, Roberts AB, Duckett CS (2004) Uncoupling of the signaling and caspase-inhibitory properties of X-linked inhibitor of apoptosis. J Biol Chem 279(10):9023–9029. https://doi.org/10.1074/jbc.M312891200
Vaux DL, Silke J (2005) IAPs, RINGs and ubiquitylation. Nat Rev Mol Cell Biol 6(4):287–297. https://doi.org/10.1038/nrm1621
Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E, Arora V, Mak TW, Lacasse EC, Waring J, Korneluk RG (2008) Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci USA 105(33):11778–11783. https://doi.org/10.1073/pnas.0711122105
Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K, Fairbrother WJ, Vucic D (2008) c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem 283(36):24295–24299. https://doi.org/10.1074/jbc.C800128200
Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, He J, Shiba T, Yang X, Yeh WC, Mak TW, Korneluk RG, Cheng G (2008) Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol 9(12):1371–1378. https://doi.org/10.1038/ni.1676
Acknowledgements
We thank Miss. Yue Zhang (Brown University) for language editing. This work was supported by research grants from the National Natural Science Foundation of China (Nos. 81471551 and 81630054).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All animal experiments are in accordance with International Guidelines and Protocols and approved by the Institutional Animal Care and Use Committee at the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, Q., Gu, L., Liao, S. et al. NG25, a novel inhibitor of TAK1, suppresses KRAS-mutant colorectal cancer growth in vitro and in vivo. Apoptosis 24, 83–94 (2019). https://doi.org/10.1007/s10495-018-1498-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-018-1498-z