Abstract
Changes in tick distribution and abundance are among the main drivers of the (re)emergence of transmitted pathogens. We aimed to uncover the reasons of the reported spread of Dermacentor reticulatus in Poland using a variety of proxies of environmental features, ground-measured temperature and remotely-sensed data of temperature and vegetation. Ground-measured temperature was recorded in 2013–2014, in sites where D. reticulatus presence (n = 16) or absence (n = 16) was confirmed. We specifically aimed to test whether changes in phenology of vegetation and the length of the growing season were correlated with field-derived data regarding the presence/absence of D. reticulatus. We also used categorical descriptions of the habitat to capture the vegetation patterns that might delineate the distribution of the tick. We demonstrated that temperature, phenology of vegetation and length of growing season have no correlation with the occurrence of D. reticulatus in Poland. There is, however, a clear association between the trends of the loss of forests and the presence of the tick. This parameter was two times higher at sites colonized by D. reticulatus in comparison to the sites where the population of the tick is not yet established. A spatial analysis demonstrated that the preferred territories for D. reticulatus are those of a highly fragmented landscape within a large patch of homogeneous vegetation, in the vicinity of permanent water courses or reservoirs.
Similar content being viewed by others
Introduction
The emergence and re-emergence of tick-borne diseases (TBDs) pose a significant threat to both human and animal health (Jongejan and Uilenberg 2004; Solano-Gallego and Baneth 2011; Bajer et al. 2014). Appearance of a new TBD in non-endemic regions is often the first sign of the extending geographical range of a particular tick species (Nijhof et al. 2007; Schaarschmidt et al. 2013). Therefore, forecasting and early detection of the spread of ticks to new areas are needed for the prevention of TBDs (Otranto and Wall 2008).
Exophilous tick species spend most of their life questing for hosts in the environment and their survival is strictly dependent on abiotic conditions (Randolph 2009). A set of adequate conditions, delineated by microclimatic conditions such as slope and aspect, snow cover, vegetation, litter layer, humus, and underlying soils provides shelter for ticks during quiescence and diapausing and is appropriate for questing (Estrada-Peña 2008). All these variables are specific for each tick species. Completion of the life cycle and tick spread rely also on host availability, thus involving specific landscape and climatic conditions for vertebrates (Estrada-Peña and de la Fuente 2014). It is vital to capture the environmental features to which ticks are associated, given that the presence of probable suitable foci and spread of a particular tick species to non-endemic regions, could be modelled based on the features of preferred habitats (Kalluri et al. 2007; Eisen and Eisen 2008). For this purpose remotely-sensed (RS) data available on relatively long time scales have been used. However, they are commonly insufficient to describe the microclimatic conditions under which ticks thrive. The use of field-recorded environmental conditions (i.e. temperature, humidity) increases significantly the reliability of the analyses (Ogden et al. 2006a; Estrada-Peña 2008). These measures have been extensively used in the modelling of epidemiological risk related to ticks feeding on humans (Ogden et al. 2005; Hoch et al. 2010; Hancock et al. 2011).
Considerable attention has been devoted to Dermacentor reticulatus (Fabricius) in Europe in the last two decades (Dautel et al. 2006; Bullová et al. 2009; Dobec et al. 2009; Hornok and Farkas 2009; Cochez et al. 2012; Halos et al. 2013; Schaarschmidt et al. 2013; René-Martellet et al. 2015; Olivieri et al. 2016), given that it is the main vector of Babesia canis (Bourdoiseau 2006; Beelitz et al. 2012). This tick species may also constitute possible threat to public health as vector of tick-borne encephalitis virus (Wójcik-Fatla et al. 2011; Mierzejewska et al. 2015a) and bacteria of genus Rickettsia (Špitalská et al. 2012; Földvári et al. 2013). Poland is one of the areas of recent expansion of D. reticulatus. Until the end of the 1980’s, this species was recorded sporadically and was present in several restricted foci in eastern regions of the country (Lachmajer 1963; Szymański 1986; Siuda 1993). In the 1990’s, D. reticulatus became common in central Poland, including the area of the capital city, Warsaw (Karbowiak 2009; Zygner and Wedrychowicz 2006; Zygner et al. 2009), resulting in outbreaks of canine babesiosis in the region (Sobczyk et al. 2005; Welc-Falęciak et al. 2009). Until now this territory is considered as endemic for canine babesiosis (Adaszek et al. 2011; Bajer et al. 2014). The range of this tick species continued to expand during the last 20 years, crossing the Vistula River barrier (Karbowiak 2009, 2014; Zygner et al. 2009; Welc-Faleciak et al. 2009; Bajer et al. 2014). In endemic regions (central and eastern Poland) D. reticulatus is the dominant species of the tick fauna on pets and livestock, with a prevalence over 80% (Mierzejewska et al. 2015b). In 2010, the first focus of D. reticulatus was confirmed in western Poland (Nowak 2011) but the species had already been collected from animals in the Dolnośląskie (western Poland) and Pomeranian (northern Poland) voivodships (Fryderyk 1998; Karbowiak and Kiewra 2010). The occurrence of D. reticulatus in these regions was later confirmed in additional isolated locations (Kadulski and Izdebska 2009; Karbowiak and Kiewra 2010).
During the period of 2012–2014, we performed a large-scale active survey of D. reticulatus in the area between the Vistula River and the western border of Poland (Mierzejewska et al. 2016), where several new foci of D. reticulatus were found. We have confirmed the expansion of the tick range and noticed the presence of a large area between the Vistula and Oder Rivers which remains free of this tick species (the gap). In another study we have demonstrated that fallow lands (abandoned crop fields and meadows) are the preferred habitats for D. reticulatus, with tick densities up to eight times higher than in cultivated pastures and meadows (Mierzejewska et al. 2015c). The tick prefers open habitats close to periodically flooded zones in valleys of rivers and streams (Siuda 1993; Zygner et al. 2009; Široký et al. 2011).
It is however unknown what factors promote the spread of D. reticulatus tick and why some areas remain uncolonized, even if located within the invaded region. In this study we aimed to determine the features that shaped the recorded distribution of D. reticulatus in Poland. We compared actual (years 2013–2014) ground temperature profiles between sites where the tick is present (tick-positive site) or absent (tick-negative site). We also analysed long-term changes in environmental features (i.e. temperature, humidity, Normalized Difference Vegetation Index, forest habitat losses) between the tick-positive and the tick-negative sites, using RS data.
Materials and methods
All analyses were performed based on surveys carried out on 54 tick-positive and 74 tick-negative sites in endemic regions, the zones of expansion and the gap between endemic and colonized sites (Fig. 1a). We used the results of a survey of D. reticulatus in Poland, performed in 2012–2014. The survey (selection of tick collection sites and their locations) has been described in detail in Mierzejewska et al. (2016). Nine tick-positive sites were situated in the endemic territory: four in eastern (Warmińsko-Mazurskie) and five in central (Mazovia) Poland. Twenty-one sites were located on the western side of the Vistula River, in the zone of expansion (ZE) of the tick. There were 24 sites in western Poland, including two in the Lubuskie focus described by Nowak (2011). The sites negative for D. reticulatus were located in both the ZE and the gap.
Weather conditions among the tick-positive and tick-negative sites
Temperature at ground level
A total of 32 loggers were used for the measurement of ground temperature (HOBO U23-001 Pro v2 Temperature/RH Data Logger, Onset Computer Corporation, USA). Twenty-five loggers were regularly distributed in the ZE and the gap (Fig. 1b). Seven additional loggers were placed in the endemic region on the eastern side of the Vistula River. In 16 of these 32 sites, the presence of D. reticulatus was confirmed. Loggers were buried 5 cm below ground level in perforated plastic boxes, used for protection. The temperature was recorded four times a day (at 04:00, 10:00, 16:00 and 22:00) from the middle of November 2012 to the beginning of July 2014. We first compared the monthly average temperature among the tick-positive and negative sites. Similar comparisons were also done with the total amount of temperature accumulated in monthly calendar intervals. For both analyses we used nested ANOVA (JMP V9.0 software) to test for statistically significant differences between the tick-positive and negative sites for any specific month, given that climatic conditions may have varied effects on ticks at specific life cycle stages (Zahler and Gothe 1995a, b; Randolph 2009).
Remotely sensed data
We used RS data to search for differences in the long-term trends of environmental features between the tick-negative and the tick-positive sites. A set of Fourier-transformed RS covariates (Estrada-Peña et al. 2014) was used to test differences in land surface temperature during the day (LSTD) and night (LSTN) as well as in the vegetation (Normalized Difference Vegetation Index, NDVI) values between the tick-positive and tick-negative sites. NDVI is a measure of the photosynthetic activity of the vegetal canopy (Wan et al. 2004) that is related to the transpiration of the plant and to its water contents. Monthly data on LSTD, LSTN, and NDVI at a spatial resolution of 0.05° were obtained from the MODIS website (https://lpdaac.usgs.gov/products/modis_products_table, accessed in December 2013). The data covered the period of 2001–2012. A complete explanation of the procedures for harmonic regression and extraction of the coefficients, as well as their improved performance over monthly raw series of RS data were provided earlier (Estrada-Peña et al. 2014). The coefficients were calculated separately for LSTD, LSTN, and NDVI, and therefore three different multifactorial ANOVA analyses were carried out to check for consistent differences in long-term RS values recorded at the tick-positive or negative sites. Given that this is a spatial analysis, point coordinates of the surveyed sites are not adequate. We therefore drew hexagons with a radius of 5 km around each collection site, and remotely-sensed features overlying each hexagon were averaged for every collection site. This set of hexagons was used for the further spatial analysis described below.
Vegetation phenology
Vegetation, land cover and its trend
We explored if medium-term changes in vegetation phenology exist between the tick-positive and negative sites on the basis that significant differences of the trends of vegetation would likely reflect different environmental conditions for D. reticulatus. We used the Global Inventory Modelling and Mapping Studies (GIMMS) NDVI data set obtained from http://ecocast.arc.nasa.gov/data/pub/gimms/. The attributes of the data set were as follows: 15-day temporal frequency, 250 m spatial resolution and a temporal span from January 1982 to December 2006. This is the latest available update of the dataset; information between 2007 and 2014 is not yet available. We examined the inflection points of the complete GIMMS time series to examine where inflection points exist using the package “cpm” (Ross 2013; http://cran.r-project.org/src/contrib/Archive/cpm/, accessed in January 2014) for the R programming environment (R Core Team 2014). We assessed significant differences in the inflection points of the NDVI series between the tick-positive and negative sites, using ANOVA as in the previous analysis.
Changes in the length of the growing season
The GIMMS NDVI data set was used to assess changes in the length of the growing season. It has been demonstrated that the success of colonisation by D. reticulatus may be highly dependent on the length of the oviposition period, which happens in spring and summer (Zahler and Gothe 1995a, b). Thus, the fitness in the oviposition period could account for the success of tick colonisation (Dautel et al. 2006) because the longer the growing season, the higher the temperatures (Jaenson and Lindgren 2011), and therefore the better conditions for the oviposition by the tick. We checked for significant differences in the duration of the growing season between the tick-positive and negative sites, using the package “changepoint” (Killick and Eckley 2014) for R. The hypothesis is that the length of the growing season for the complete series 1982–2006 would show significantly different values between the tick-positive and negative sites. Because of the uncertainties in this analysis, we completed it in two different ways: one which included the number of 15-day periods between spring and autumn, and the other including only the number of 15-day periods of summer. Next, we analysed the amount of the NDVI accumulated between the beginning and the end of the considered seasons, looking for further significant differences between the tick-positive and negative sites, using, as before an multifactorial ANOVA.
Changes of landscape as associated with the presence/absence of D. reticulatus
Ticks are sensitive to changes in the landscape (Estrada-Peña 2003; Ostfeld et al. 2005). This is sometimes complex to evaluate because ticks are not associated to pre-tailored categories of vegetation, that are based on purely botanical terms (Gilot et al. 1979; Hubálek et al. 2003). Therefore, we used a combined analysis involving both the changes in forest areas and in the types of vegetation, adhering to the methods explained below.
Vegetation Continuous Fields
We used the Vegetation Continuous Fields (VCF) dataset (DiMiceli et al. 2011), for the period 2000–2010 at a spatial resolution of 250 m (available at http://glcf.umd.edu/data/vcf/, accessed in December 2013) to test for changes of “forest” or “grassland” in the target territory. Given that D. reticulatus prefers open sites, we tested for differences in VCF trends between the tick-positive and negative sites, which should ascertain trends in the loss of forest habitats. Trends for the VCF series and statistically significant differences were computed as earlier.
Spatial analysis of landscape categories: High-resolution habitat categories
We assessed if D. reticulatus is associated with a range of categories of vegetation, as defined by harmonised high-resolution datasets, and if the differences between the tick-positive and negative sites are statistically significant. We used the GlobCover dataset, produced by the European Space Agency (Bicheron et al. 2006, available at http://www.gofcgold.wur.nl/sites/globcover.php, accessed in December 2013). It is a set of 40 categories of vegetation at a resolution of 300 m, developed from the MERIS instrument on board the ENVISAT series of satellites. Attempts to use the CORINE dataset produced unreliable results, because such a dataset is currently available only at level 3 for Poland. The low number of landscape categories associated with CORINE 3 resulted in an insufficient description of the preferred habitat for D. reticulatus.
We associated the type of dominant vegetation, the fragmentation (number of different categories of vegetation) and the distance to either water courses or reservoirs to the tick-positive or negative sites. This is in part a qualitative assessment (as opposed to quantitative) of the preferred habitat of the tick, and therefore statistical tests accounting for quantitative variables cannot be applied. We therefore used a recursive partitioning method to uncover the set of variables that better split the surveyed points into the tick-positive or the tick-negative sites. The method recursively partitions data according to relationships between the explanatory variables and the response variable, here the tick-positive/negative site, creating a tree of partitions. It finds a set of cuts or groupings of X values that best predict an Y value. It does this by exhaustively searching all possible cuts or groupings. These partitions of the data are done recursively, forming a tree of decision rules until the desired fit is reached. Node splitting is based on the LogWorth statistic (Gaudard et al. 2006). After the algorithm recursively splits all the possible combinations of the explanatory variables, a final decision tree is available, which displays the partitions and the best combination of variables that separate the tick-positive or negative sites for D. reticulatus. We used a nested ANOVA to check for statistical differences between the different type of vegetation (using the mentioned different datasets) in the surveyed territory.
Results
Effect of weather features on the successful colonisation by D. reticulatus
Temperature at ground level
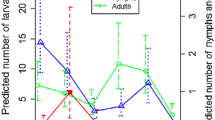
We found no significant differences in the annual temperature profiles at ground level between the tick-positive and negative sites. In fact, the monthly average temperature for both site categories overlapped (Fig. 2a).
A comparative overview of the main quantitative traits measured to ascertain the distribution of Dermacentor reticulatus. a Values of the monthly accumulated temperatures (°C) over the year for the D. reticulatus positive and negative sites and average of monthly accumulated NDVI values in the period 1982–2006 for D. reticulatus positive and negative sites in the target territory. Regarding NDVI, the chart shows the accumulated values of NDVI in the periods which were detected as belonging to “summer” or to “spring to autumn” by an algorithm that explores the inflection points of the time series of data. Original data were obtained from the GIMMS-NDVI dataset. b Number of 15-day intervals which were detected as belonging to “summer” or to “spring to autumn”. Data regarding NDVI were obtained from the Global Inventory Modelling and Mapping Studies (GIMMS)-NDVI dataset. c Differences in the trend of vegetation fields values between positive and negative sites for D. reticulatus. The index indicates the “amount of forest”, therefore, the lower the value, the highest the trend in the time for vegetation stands belonging to the category “forest” that were transformed into “open vegetation” in the period of time
Remotely sensed data
The analyses of Fourier-transformed RS covariates for LSTD, LSTN and NDVI in a 12-year time span did not show any significant differences between the tick-positive and negative sites (p = 0.37 for all the variables).
Vegetation phenology
Land cover and its trend using the GIMMS-NDVI data set
The NDVI calculated for the 25-year data set showed a general positive trend. However, differences in the NDVI trend between the tick-positive and negative sites were not significant.
Changes in the length of the growing season
No significant differences were evident in the comparison of the duration of spring–autumn, the duration of summer, the accumulated NDVI in spring–autumn and in the summer (Fig. 2b).
Changes of landscape as associated to the presence/absence of D. reticulatus
Vegetation Continuous Fields
The analysis of the trend of VCF in the period 2000–2010 showed that the loss of forest habitats for the tick-positive sites was twice as high as compared to the tick-negative-sites (Fig. 2c) (F 1,11 = 13.45; p = 0.01).
A spatial analysis of landscape qualitative categories
We assessed the reliability of the combined analysis of the diversity of vegetation, the dominant category of vegetation, and the distance to rivers or to water courses, to assess the presence or absence of D. reticulatus (Fig. 3). No single or set of variable(s) correctly classified a tick-positive or tick-negative site. The best model, measured with the Akaike Information Criterium, was 318.8, with an r-square of only 0.33 with a total of seven splits of the categorical variables. This model ascribed the majority of the records of D. reticulatus to cells in the grid displaying a large single patch of vegetation surrounded by a highly fragmented landscape, in the vicinity of a permanent water course or reservoir.
Results from a recursive algorithm that partitions categorical descriptions of the habitat, aimed to discriminate the sites positive or negative for Dermacentor reticulatus. The algorithm looks for the combination of variables that best discriminate two categories of sites and produces the most adequate separation between the tick-positive and negative sites. The algorithm is recursive and separate sites not well discriminated using further combinations of variables
Discussion
This paper explored the reliability of several vegetation and weather-derived data (including landscape classifications) to separate the sites where D. reticulatus is present or absent in a wide territory of Poland and to conclude on the possibility of further expansion. We found very few statistically significant associations, supporting the idea that the tick is colonizing pre-existing suitable habitats. Perhaps surprisingly, variables used before to describe the habitat or niche of ticks (Mejlon 1997; Vail and Smith 1998; Alekseev and Dubinina 2000; Schulze et al. 2001) did not help to define D. reticulatus distribution in Poland. Ground-measured and RS-derived temperature do not describe the pattern of occurrence of D. reticulatus in Poland.
Similar studies for D. reticulatus are scarce and focused to capture the periods of activity and abundance of the tick (Hubálek et al. 2003; Bartosik et al. 2011; Buczek et al. 2014). The comparison of features determining the limiting factors for its occurrence pattern in a large territory has never been done before. Hubálek et al. (2003) investigated the impact of microclimate in South Moravia (Czech Republic) on the adults of D. reticulatus. The soil temperature was found to be the variable with the highest importance in the predictive models of the relative abundance and host seeking activity of D. reticulatus. The temperature threshold for questing behaviour of adults was around 0.7/–0.1 °C, explaining its activity period in autumn and winter. In our study, the two series of ground-recorded and RS measurements of temperature gave coherent results, without differences between the tick-positive and negative sites. This is an important finding, given that satellite data are available for analysis since the beginning of the twenty-first century; therefore it covers the time span during the expansion of D. reticulatus in Poland. The lack of significant differences between the tick-positive and negative sites leads to a more general conclusion regarding the likely non-significant impact of temperature trends on this tick species. This underlines the need for further investigation to determine other possible reasons for the observed expansion. The main conclusion from this part of the analysis is that, irrespective of the changes in weather over the last few years, there are no significant differences between the sites colonised by ticks and those that are still free of D. reticulatus.
The analysis of a similar series of values of NDVI was unable to detect changes in the photosynthetic activity of the plants in the sites where the tick is present or absent. NDVI is a convenient surrogate to evaluate changes and trends of humidity in the vegetation canopy. NDVI is only temporally responsive to rainfall, and is more suitable for a long term measurement of water contents, which in turn is known to affect tick activity and mortality (Randolph 2000; Sonenshine and Roe 2014). NDVI has been repeatedly used to monitor the habitat of ticks (Estrada-Peña 1999; Bisanzio et al. 2008; Ogden et al. 2006b). Hubálek et al. (2003) and Buczek et al. (2014) have demonstrated the lack of impact of rainfall and wind speed on the host-seeking activity of D. reticulatus. Rainfall is unlikely to be an important factor limiting the distribution of D. reticulatus in Poland, given that annual mean precipitation is 622.8 mm (data from the Institute of Meteorology and Water Management) with the lowest values in the centre of the country (about 550 mm) and the highest in mountain regions (about 1000 mm). The optimal range of annual mean precipitation for D. reticulatus (400–1000 mm) was estimated by Gilot et al. (1973). In summary, the two most important traits of the weather, namely temperature and air water contents, did not differ between the colonised sites and those where the tick has not yet been collected in Poland.
We thus turned to examine if the growing season changed between the two categories of sites. The rationale is that changes in the duration of the growing season could be a more adequate marker of habitat suitability for D. reticulatus because a seasonal component is included. This is of importance because averaged values of weather variables tend to obscure the importance of a given trait at critical moments of the life cycle of the tick. This approach, however, also failed to provide significant differences between the tick-positive and negative sites in the surveyed territory. While obvious changes in the duration of summer or spring–autumn were noted, as already reported (Fensholt et al. 2009; Fensholt and Proud 2012) they do not correspond with the pattern of occurrence of the ticks. We think that high resolution data, such as a long term series of Landsat images, could probably increase the sensitivity in detecting changes in the trend of NDVI for tick presence/absence sites. A major limitation in the use of this approach is to cover a large territory with images that have a resolution of only 15 m, and for which a large number of images are necessary to cover the period of interest. Landsat images, however, have been used in studies defining the habitat of Amblyomma variegatum (Hugh-Jones 1991) and in delineating areas according to the force of transmission of tick-borne diseases (Daniel et al. 1998).
The results of the trends of VCF analysis indicate that this series may be a promising indicator of the habitat for D. reticulatus. This set of data is intended to deliver information about the trends of changes in forested versus open habitats. We demonstrated that the increase in the trend of the loss of forest habitats was twice as high for the tick-positive sites as for the negative ones in the complete target territory. The time span of data availability (2000–2010) was relatively short, thus greater differences between the tick-positive and the tick-negative sites are expected. Forests are most often transformed to arable land, or lost because of urbanisation or to lesser extent as a result of forest management (Firbank et al. 2008). These factors promote the creation of open habitats, preferred by D. reticulatus. The development of a mosaic landscape, in which forest has a trend to slowly disappear, would favour the occurrence of the tick both because it provides suitable set of conditions for D. reticulatus and the landscape favours the presence of hosts.
We aimed to integrate these findings into a classification of the territory that includes the amount of forest, the fragmentation of the habitat, and the distance to rivers and water courses. The latter is based on the reported findings that large vertebrates tend to use water courses for movements (Dickinson et al. 2000) and provides a proxy for “host availability” and connectivity among tick infested patches. The results showed the reliability of this approach to characterise the sites colonised by D. reticulatus. The method, however, did not give a complete and perfect separation of sites, probably due the low number of descriptive levels of the available dataset (3 levels as standard for Europe). CORINE Land Cover at 5 levels would probably increase the predictive power of this analysis, but it is currently unavailable for Poland. Results of the landscape analysis indicate that areas preferred by D. reticulatus are large patches of homogeneous vegetation surrounded by a highly fragmented landscape, in the vicinity of a permanent water course or reservoir. This is a heterogeneous mosaic that may be highly interconnected, allowing the movements of infested hosts and therefore improving the ability of the tick to colonise new sites (Li et al. 2012; Kittle 2014). A landscape in which the forest is progressively cleared and fragmented seems to be the preferred habitat for D. reticulatus, also in terms of the mobility of hosts and their essential role in the transportation of ticks (Jaenson et al. 2012; Carpi et al. 2008; Pugliese and Rosa 2008; Hoch et al. 2010). Populations of wolves and moose, which have a great mobility (Mech and Boitani 2003; Hoffman et al. 2006), have recently increased in Poland and moose is considered as one of the main hosts of D. reticulatus (Bogdaszewska 2004). Water courses support landscape connectivity serving as potential corridors for wild animal migration. Banks of rivers and canals also provide open land with shrubs and high grasses described previously as a preferred habitat of D. reticulatus (Siuda 1993; Zygner et al. 2009; Široký et al. 2011).
Conclusions
Results of this study indicate the temperature (recorded at micro and macro scale) and length of the growing season create no barrier for the spread and distribution of D. reticulatus in the territory of Poland. However the spread of D. reticulatus tick is associated with the loss of forest.
References
Adaszek Ł, Martinez AC, Winiarczyk S (2011) The factors affecting the distribution of babesiosis in dogs in Poland. Vet Parasitol 181(2–4):160–165
Alekseev AN, Dubinina HV (2000) Abiotic parameters and diel and seasonal activity of Borrelia-infected and uninfected Ixodes persulcatus (Acarina: Ixodidae). J Med Entomol 37:9–15
Bajer A, Mierzejewska EJ, Rodo A, Bednarska M, Kowalec M, Welc-Faleciak R (2014) The risk of vector-borne infections in sled dogs associated with existing and new endemic areas in Poland. Part 1: a population study on sled dogs during the racing season. Vet Parasitol 202:276–286
Bartosik K, Wisniowski L, Buczek A (2011) Abundance and seasonal activity of adult Dermacentor reticulatus (Acari: Amblyommidae) in eastern Poland in relation to meteorological conditions and the photoperiod. Ann Agric Environ Med 18:340–344
Beelitz P, Schumacher S, Marholdt F, Pfister K, Silaghi C (2012) The prevalence of Babesia canis canis in marsh ticks (Dermacentor reticulatus) in the Saarland. Berl Munch Tierarztl Wochenschr 125:168–171
Bicheron P, Leroy M, Brockmann C, Krämer U, Miras B, Huc M, Ninõ F, Defourny P, Vancutsem C, Arino O, Ranéra F, Petit D, Amberg V, Berthelot B, Gross D (2006) Globcover: a 300 m global land cover product for 2005 using ENVISAT MERIS time series. In: Proceedings of the second international symposium on recent advances in quantitative remote sensing, pp 538–542
Bisanzio D, Amore G, Ragagli C, Tomassone L, Bertolotti L, Mannelli A (2008) Temporal variations in the usefulness of normalized difference vegetation index as a predictor for Ixodes ricinus (Acari: Ixodidae) in a Borrelia lusitaniae focus in Tuscany, central Italy. J Med Entomol 45:547–555
Bogdaszewska Z (2004) Występowanie i ekologiakleszcza łąkowego Dermacentor reticulatus (Fabricius, 1794) w ognisku mazurskim. I. Określenie obecnego zasięgu występowania. Wiad Parazytol 50:727–730
Bourdoiseau G (2006) Canine babesiosis in France. Vet Parasitol 138:118–125
Buczek A, Bartosik K, Zając Z (2014) Changes in the activity of adult stages of Dermacentor reticulatus (Ixodida: Amblyommidae) induced by weather factors in eastern Poland. Parasit Vectors 7:245
Bullová E, Lukáň M, Stanko M, Peťko B (2009) Spatial distribution of Dermacentor reticulatus tick in Slovakia in the beginning of the 21st century. Vet Parasitol 165:357–360
Carpi G, Cagnacci F, Neteler M, Rizzoli A (2008) Tick infestation on roe deer in relation to geographic and remotely sensed climatic variables in a tick-borne encephalitis endemic area. Epidemiol Infect 136:1416–1424
Cochez C, Lempereur L, Madder M, Claerebout E, Simons L, De Wilde N, Linden A, Saegerman C, Heyman P, Losson B (2012) Foci report on indigenous Dermacentor reticulatus populations in Belgium and a preliminary study of associated babesiosis pathogens. Med Vet Entomol 26(3):355–358
Daniel M, Kolár J, Zeman P, PavelkaK Sádlo J (1998) Predictive map of Ixodes ricinus high-incidence habitats and a tick-borne encephalitis risk assessment using satellite data. Exp Appl Acarol 22:417–433
Dautel H, Dippel C, Oehme R, Hartelt K, Schettler E (2006) Evidence for an increased geographical distribution of Dermacentor reticulatus in Germany and detection of Rickettsia sp. RpA4. Int J Med Microbiol 296:149–156
Dickinson MH, Farley CT, Full RJ, Koehl MAR, Kram R, Lehman S (2000) How animals move: an integrative view. Science 288(5463):100–106
DiMiceli CM, Carroll ML, Sohlberg RA, Huang C, Hansen MC, Townshend JRG (2011) Vegetation continuous fields MOD44B. 20011 percent tree cover. Collection 5. University of Maryland, College Park, MD
Dobec M, Golubic D, Punda-Polic V, Kaeppeli F, Sievers M (2009) Rickettsia helvetica in Dermacentor reticulatus ticks. Emerg Infect Dis 15:98–100. doi:10.3201/eid1501.080815
Eisen RJ, Eisen L (2008) Spatial modeling of human risk of exposure to vector-borne pathogens based on epidemiological versus arthropod vector data. J Med Entomol 45:181–192
Estrada-Peña A (1999) Geostatistics as predictive tools to estimate Ixodes ricinus (Acari: Ixodidae) habitat suitability in the western Palearctic from AVHRR satellite imagery. Exp Appl Acarol 23:337–349
Estrada-Peña A (2003) The relationships between habitat topology, critical scales of connectivity and tick abundance Ixodes ricinus in a heterogeneous landscape in northern Spain. Ecography 26:661–671
Estrada-Peña A (2008) Climate, niche, ticks, and models: what they are and how we should interpret them. Parasitol Res 103:87–95
Estrada-Peña A, de la Fuente J (2014) The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral Res 108:104–128
Estrada-Peña A, Estrada-Sánchez A, de la Fuente J (2014) A global set of Fourier-transformed remotely sensed covariates for the description of abiotic niche in epidemiological studies of tick vector species. Parasit Vectors 7:302
Fensholt R, Proud SR (2012) Evaluation of earth observation based global long term vegetation trends—comparing GIMMS and MODIS global NDVI time series. Remote Sens Environ 119:131–147
Fensholt R, Rasmussen K, Nielsen TT, Mbow C (2009) Evaluation of earth observation based long term vegetation trends—intercomparing NDVI time series trend analysis consistency of Sahel from AVHRR GIMMS, Terra MODIS and SPOT VGT data. Remote Sens Environ 113:1886–1898
Firbank LG, Petit S, Smart S, Blain A, Fuller RJ (2008) Assessing the impacts of agricultural intensification on biodiversity: a British perspective. Philos Trans R Soc Lond B Biol Sci 363:777–787
Földvári G, Rigó K, Lakos A (2013) Transmission of Rickettsia slovaca and Rickettsia raoultii by male Dermacentor marginatus and Dermacentor reticulatus ticks to humans. Diagn Microbiol Infect Dis 76:387–389
Fryderyk S (1998) Nowe interesujące stwierdzenie Dermacentor reticulatus [FABR.] [Acari, Ixodidae] na dziku [Sus scrofa L.]. Wiad Parazytol 44:737–739
Gaudard M, Ramsey P, Stephens M (2006) Interactive data mining and design of experiments: the JMP® partition and custom design platforms. North Haven Group LLC, Cary North Carolina
Gilot B, Pautou G, ImmlerR Moncada E (1973) Suburban biotype of Dermacentor reticulatus (Fabricius, 1794) (Ixodoidea). Preliminary study. Revue suisse de zoologie; annales de la Societe zoologique suisse et du Museum d’histoire naturelle de Geneve 80(2):411–430
Gilot B, Pautou G, Moncada E, Lachet B, Christin JG (1979) La cartographie des populations de tiquesexophiles par le biais de la végétation: bases écologiques, intérêtépidémiologique. Doc de Cart Ecol 22:65–80
Halos L, Lebert I, Chao I, Vourc’h G, Ducrot C, Abrial D, Ravier JF, Guillot J (2013) Questionnaire-based survey on distribution and clinical incidence of canine babesiosis in France. BMC Vet Res 9:41. doi:10.1186/1746-6148-9-41
Hancock PA, Brackley R, Palmer SC (2011) Modelling the effect of temperature variation on the seasonal dynamics of Ixodes ricinus tick populations. Int J Parasitol 41:513–522
Hoch T, Monnet Y, Agoulon A (2010) Influence of host migration between woodland and pasture on the population dynamics of the tick Ixodes ricinus: a modelling approach. Ecol Model 221:1798–1806
Hoffman JD, Genoways HH, Choate JR (2006) Long-distance dispersal and population trends of moose in the central United States. Alces 42:115–131
Hornok S, Farkas R (2009) Influence of biotope on the distribution and peak activity of questing ixodid ticks in Hungary. Med Vet Entomol 23:41–46
Hubálek Z, Halouzka J, Juricova Z (2003) Host-seeking activity of ixodid ticks in relation to weather variables. J Vector Ecol 28:159–165
Hugh-Jones M (1991) The remote recognition of tick habitats. J Agric Entomol 8:309–315
Jaenson TG, Lindgren E (2011) The range of Ixodes ricinus and the risk of contracting Lyme borreliosis will increase northwards when the vegetation period becomes longer. Ticks Tick Borne Dis 2:44–49. doi:10.1016/j.ttbdis.2010.10.006
Jaenson TG, Jaenson DG, Eisen L, Petersson E, Lindgren E (2012) Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit Vectors 10(5):8. doi:10.1186/1756-3305-5-8
Jongejan F, Uilenberg G (2004) The global importance of ticks. Parasitology 129:S3–S14
Kadulski S, Izdebska JN (2009) New data on distribution of Dermacentor reticulatus (Fabr.) (Acari, Ixodidae) in Poland. In: Buczek A, Błaszak Cz (eds) Stawonogi. Inwazje i ich ograniczanie. Akapit, Lublin, pp 53–58
Kalluri S, Gilruth P, Rogers D, Szczur M (2007) Surveillance of arthropod vector-borne infectious diseases using remote sensing techniques: a review. PLoS Pathog 3:e116
Karbowiak G (2009) Kleszcz łąkowy Dermacentor reticulatus - występowanie, biologa i rola jako wektora chorób odkleszczowych. Habilitation thesis. Instytut Parazytologii im. Witolda Stefańskiego PAN, Warsaw
Karbowiak G (2014) The occurrence of the Dermacentor reticulatus tick—its expansion to new areas and possible causes. Ann Parasitol 60:37–47
Karbowiak G, Kiewra D (2010) New locations of Dermacentor reticulatus ticks in western Poland: the first evidence of the merge in D. reticulatus occurrence areas. Wiad Parazytol 56:333–340
Killick R, Eckley IA, (2014) changepoint: an R package for changepoint analysis. J Stat Softw 58:1–19. http://www.jstatsoft.org/v58/i03/
Kittle AM (2014) The influence of prey availability, habitat attributes and other factors on space use by social carnivores. Doctoral dissertation, The University of Guelph
Lachmajer J (1963) Stan badań nad pasożytniczymi Arthropoda w Polsce. Wiad Parazytol 9:359–369
Li S, Hartemink N, Speybroeck N, Vanwambeke SO (2012) Consequences of landscape fragmentation on Lyme disease risk: a cellular automata approach. PLoS ONE 7(6):e39612. doi:10.1371/journal.pone.0039612
Mech LD, Boitani L (2003) Wolves-behavior, ecology, and conservation. University of Chicago, Chicago
Mejlon HA (1997) Diel activity of Ixodes ricinus (Acari: Ixodidae) at two locations near Stockholm, Sweden. Exp Appl Acarol 21:247–255
Mierzejewska EJ, Pawełczyk A, Radkowski M, Welc-Falęciak R, Bajer A (2015a) Pathogens vectored by the tick, Dermacentor reticulatus, in endemic regions and zones of expansion in Poland. Parasit Vectors 8:490
Mierzejewska EJ, Welc-Faleciak R, Karbowiak G, Kowalec M, Behnke JM, Bajer A (2015b) Dominance of Dermacentor reticulatus over Ixodes ricinus (Ixodidae) on livestock, companion animals and wild ruminants in eastern and central Poland. Exp Appl Acarol 66:83–101
Mierzejewska EJ, Alsarraf M, Behnke JM, Bajer A (2015c) The effect of changes in agricultural practices on the density of Dermacentor reticulatus ticks. Vet Parasitol 211:259–265. doi:10.1016/j.vetpar.2015.05.023
Mierzejewska EJ, Estrada-Peña A, Alsarraf M, Kowalec M, Bajer A (2016) Mapping of Dermacentor reticulatus expansion in Poland in 2012–2014. Ticks Tick Borne Dis 7(1):94–106
Nijhof AM, Bodaan C, Postigo M, Nieuwenhuijs H, Opsteegh M, Franssen L, Jebbink F, Jongejan F (2007) Ticks and associated pathogens collected from domestic animals in the Netherlands. Vector Borne Zoonotic Dis 7(4):585–595
Nowak M (2011) Discovery of Dermacentor reticulatus (Acari: Amblyommidae) populations in the Lubuskie Province (Western Poland). Exp Appl Acarol 54:191–197
Ogden NH, Bigras-Poulin M, O’Callaghan CJ, Barker IK, Lindsay LR, Maarouf A, Smoyer-Tomic KE, Waltner-Toews D, Charron D (2005) A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int J Parasitol 35:375–389
Ogden NH, Barker IK, Beauchamp G, Brazeau S, Charron DF, Maarouf A, Morshed MG, O’Callaghan CJ, Thompson RA, Waltner-Toews D, Waltner-Toews M, Lindsay LR (2006a) Investigation of ground level and remote-sensed data for habitat classification and prediction of survival of Ixodes scapularis in habitats of south eastern Canada. J Med Entomol 43:403–414
Ogden NH, Trudel L, Artsob H, Barker IK, Beauchamp G, Charron DF, Drebot MA, Galloway TD, O’Handley R, Thompson RA, Lindsay LR (2006b) Ixodes scapularis ticks collected by passive surveillance in Canada: analysis of geographic distribution and infection with Lyme borreliosis agent Borrelia burgdorferi. J Med Entomol 43:600–609
Olivieri E, Zanzani SA, Latrofa MS, Lia RP, Dantas-Torres F, Otranto D, Manfredi MT (2016) The southernmost foci of Dermacentor reticulatus in Italy and associated Babesia canis infection in dogs. Parasit Vectors 18:9–213. doi:10.1186/s13071-016-1502-9
Ostfeld RS, Glass GE, Keesing F (2005) Spatial epidemiology: an emerging (or re-emerging) discipline. Trends Ecol Evol 20:328–336
Otranto D, Wall R (2008) New strategies for the control of arthropod vectors of disease in dogs and cats. Med Vet Entomol 22:291–302
Pugliese A, Rosa R (2008) Effect of host populations on the intensity of ticks and the prevalence of tick-borne pathogens: how to interpret the results of deer exclosure experiments. Parasitology 135:1531–1544
Randolph SE (2000) Ticks and tick-borne disease systems in space and from space. Adv Parasitol 47:217–243
Randolph SE (2009) Epidemiological consequences of the ecological physiology of ticks. Adv Insect Phys 37:297–339
René-Martellet M, Moro CV, Chêne J, Bourdoiseau G, Chabanne L, Mavingui P (2015) Update on epidemiology of canine babesiosis in Southern France. BMC Vet Res 25(11):223. doi:10.1186/s12917-015-0525-3
Ross SM (2013) Applied probability models with optimization applications. Courier Corporation, North Chelmsford
Schaarschmidt D, Gilli U, Gottstein B, Marreros N, Kuhnert P, Daeppen JA, Rosenberg G, Hirt D, Frey CF (2013) Questing Dermacentor reticulates harbouring Babesia canis DNA associated with outbreaks of canine babesiosis in the Swiss Midlands. Ticks Tick Borne Dis 4:334–340
Schulze TL, Jordan RA, Hung RW (2001) Effects of selected meteorological factors on diurnal questing of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J Med Entomol 38:318–324
Široký P, Kubelová M, Bednář M, Modrý D, Hubálek Z, Tkadlec E (2011) The distribution and spreading pattern of Dermacentor reticulatus over its threshold area in the Czech Republic—how much is range of this vector expanding? Vet Parasitol 183:130–135
Siuda K (1993) Kleszcze Polski (Acari: Ixodida): Systematyka i rozmieszczenie. Polskie Towarzystwo Parazytologiczne, Warszawa
Sobczyk AS, Kotomski G, Gorski P, Wedrychowicz H (2005) Usefulness of touch-down PCR assay for the diagnosis of atypical cases of Babesia canis canis infections in dogs. Bull Vet Inst Pulawy 49(4):407
Solano-Gallego L, Baneth G (2011) Babesiosis in dogs and cats—expanding parasitological and clinical spectra. Vet Parasitol 181:48–60
Sonenshine DE, Roe RM (2014) Biology of ticks, vol 1. Oxford University Press, New York, pp 12–13
Špitalská E, Stefanidesová K, Kocianová E, Boldiš V (2012) Rickettsia slovaca and Rickettsia raoultii in Dermacentor marginatus and Dermacentor reticulatus ticks from Slovak Republic. Exp Appl Acarol 57:189–197
Szymański S (1986) Distribution of the tick Dermacentor reticulatus (Fabricius, 1794) (Ixodidae) in Poland. Acta Parasitol Polonica 31:143–154
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Vail SG, Smith G (1998) Air temperature and relative humidity effects on behavioral activity of blacklegged tick (Acari: Ixodidae) nymphs in New Jersey. J Med Entomol 35:1025–1028
Wan Z, Wang P, Li X (2004) Using MODIS land surface temperature and normalized difference vegetation index products for monitoring drought in the southern Great Plains, USA. Int J Remote Sens 25:61–72
Welc-Falęciak R, Rodo A, Siński E, Bajer A (2009) Babesia canis and other tick-borne infections in dogs in Central Poland. Vet Parasitol 166:191–198
Wójcik-Fatla A, Cisak E, Zając V, Zwoliński J, Dutkiewicz J (2011) Prevalence of tick-borne encephalitis virus in Ixodes ricinus and Dermacentor reticulatus ticks collected from the Lublin region (eastern Poland). Ticks Tick Borne Dis 2:16–19
Zahler M, Gothe R (1995a) Effect of temperature and humidity on egg hatch, moulting and longevity of larvae and nymphs of Dermacentor reticulatus (Ixodidae). Appl Parasitol 36:53–65
Zahler M, Gothe R (1995b) Effect of temperature and humidity on longevity of unfed adults and on oviposition of engorged females of Dermacentor reticulatus (Ixodidae). Appl Parasitol 36:200–211
Zygner W, Wedrychowicz H (2006) Occurrence of hard ticks in dogs from Warsaw area. Ann Agric Environ Med 13:355–359
Zygner W, Górski P, Wędrychowicz H (2009) New localities of Dermacentor reticulatus tick (vector of Babesia canis canis) in central and eastern Poland. Pol J Vet Sci 12:549–555
Acknowledgements
The study was funded by the National Science Centre (NCN) Grant OPUS 2011/03/B/NZ8/02212 and partially supported by the National Science Center (NCN) Grant Sonata Bis 2014/14/E/NZ7/00153.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mierzejewska, E.J., Estrada-Peña, A. & Bajer, A. Spread of Dermacentor reticulatus is associated with the loss of forest area. Exp Appl Acarol 72, 399–413 (2017). https://doi.org/10.1007/s10493-017-0160-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-017-0160-8