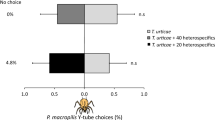
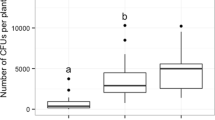
Abstract
To determine whether to use single or multiple predator species for biological pest control requires manipulative field experiments. We performed such tests in Benin (West Africa) in cassava fields infested by the cassava green mite Mononychellus tanajoa, and the cotton red mite Oligonychus gossypii. These fields also harboured the cassava apex-inhabiting predator Typhlodromalus aripo and either the leaf-inhabiting predator Amblydromalus manihoti or Euseius fustis. We manipulated predator species composition on individual plants to determine their effect on prey and predator densities. In fields with T. aripo plus A. manihoti, M. tanajoa densities were reduced by T. aripo alone or together with A. manihoti, but neither of these predators, alone or together, reduced O. gossypii densities. In fields with T. aripo plus E. fustis, T. aripo alone or together with E. fustis exerted significant control over O. gossypii, but weak control over M. tanajoa. Densities of any of the predator species were not affected by co-occurring predator species, suggesting a minor role for intraguild predation in the field, contrary to earlier experiments on small plants in the laboratory. We conclude that (1) T. aripo is the most effective predator species in suppressing M. tanajoa, (2) two predator species, T. aripo and E. fustis, are needed to suppress O. gossypii, and (3) predator species together on the same plant do not negatively affect each other nor the extent to which they control their prey. We argue that intraguild predation is reduced due to partial niche separation among predator species.





Similar content being viewed by others
References
Bakker FM, Klein ME (1992) Transtrophic interactions in cassava. Exp Appl Acarol 14:293–311
Bonato O, Baumgartner J, Gutierrez J (1995a) Comparison of biological and demographic parameters for Mononychellus progresivus and Oligonychus gossypii on cassava: influence of temperature. Entomol Exp Appl 75:119–125
Bonato O, Baumgartner J, Gutierrez J (1995b) Sampling plans for Mononychellus progresivus and Oligonychus gossypii (Acari: Tetranychidae) on cassava in Africa. J Econ Entomol 88:1296–1300
Bonato O, Da S, Noronha AC, De Moraes GJ (1999) Distribution et échantillonnage des populations de Amblyseius manihoti Moraes (Acari, Phytoseiidae) sur manioc au Brésil. J Appl Entomol 123:541–546
Briggs CJ (1993) Competition among parasitoid species on a stage-structured host and its effect on host suppression. Am Nat 141:372–397
Bruce-Oliver SJ, Hoy MA, Yaninek JS (1996) Effect of some food sources associated with cassava in Africa on the development, fecundity and longevity of Euseius fustis (Pritchard and Baker) (Acari: Phytoseiidae). Exp Appl Acarol 20:73–85
Cardinale BJ, Srivastava DS, Duffy JE, Wright JP, Downing AL, Sankaran M, Jouseau C (2006) Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443:989–992
Chant DA, McMurtry JA (2005) A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): part VI. The tribe Euseiini n. tribe, subtribes Typhlodromalina n. subtribe, Euseiina n. subtribe, and Ricoseiina n. subtribe. Int J Acarol 31:187–224
Denoth M, Frid L, Myers JH (2002) Multiple agents in biological control: improving the odds? Biol Control 24:20–30
Finke DL, Snyder WE (2008) Niche partitioning increases resource exploitation by diverse communities. Science 321:1488–1490
Gnanvossou D, Hanna R, Dicke M (2002) Prey-related odor preference of the predatory mites Typhlodromalus manihoti and Typhlodromalus aripo (Acari: Phytoseiidae). Exp Appl Acarol 27:39–56
Gnanvossou D, Yaninek JS, Hanna R, Dicke M (2003a) Effects of prey mite species on life history of the phytoseiid predators Typhlodromalus manihoti and Typhlodromalus aripo. Exp Appl Acarol 30:265–278
Gnanvossou D, Hanna R, Dicke M (2003b) Infochemical-mediated niche use by the predatory mites Typhlodromalus manihoti and T. aripo (Acari: Phytoseiidae). J Insect Behav 16:523–535
Gnanvossou D, Hanna R, Dicke M (2003c) Infochemical-mediated intraguild interactions among three predatory mites on cassava plants. Oecologia 135:84–90
Gnanvossou D, Hanna R, Yaninek JS, Toko M (2005) Comparative life history traits of three neotropical phytoseiid mites maintained on plant-based diets. Biol Control 35:32–39
Hanna R, Yaninek JS, Megevand B, Toko M, Onzo A, Gnanvossou D, Zannou I, Paraiso G (1998) Biological control of cassava green mite in Africa—status and outlook. In: Proceedings of the 8th triennial symposium of the International Society of Tropical Root Crops-Africa Branch, Oct 1998, Cotonou, Bénin
Hanna R, Onzo A, Lingeman R, Yaninek JS, Sabelis MW (2005) Seasonal cycles and persistence in an acarine predator–prey system on cassava in Africa. Pop Ecol 47:107–117
Littell RC, Pendergast J, Natarajan R (2000) Tutorial in biostatistics: modelling covariance structure in the analysis of repeated measures data. Stat Med 19:1793–1819
Losey JE, Denno RF (1998) Positive predator–predator interactions: enhanced predation rates and synergistic suppression of aphid populations. Ecology 79:2143–2152
Losey JE, Denno RF (1999) Factors facilitating synergistic predation: the central role of synchrony. Ecol Appl 9:378–386
Magalhães S, Bakker FM (2002) Plant feeding by a predatory mite inhabiting cassava. Exp Appl Acarol 27:27–37
Magalhães S, Janssen A, Hanna R, Sabelis MW (2002) Flexible antipredator behaviour in herbivorous mites through vertical migration in a plant. Oecologia 132:143–149
Magalhães S, Brommer JE, Silva ES, Bakker FM, Sabelis MW (2003) Life-history trade-off in two predator species sharing the same prey: a study on cassava-inhabiting mites. Oikos 102:533–542
Moraes GJ, Zannou ID, Oliveira AR, Yaninek JS, Hanna R (2006) Phytoseiid mites of the subtribes Typhlodromalina and Euseiina (Acari: Phytoseiidae: Euseiini) from sub-Saharan Africa. Zootaxa 1114:1–52
Northfield TD, Snyder WE, Snyder GB, Eigenbrode SD (2012) A simple plant mutation abets a predator-diversity cascade. Ecology 93:411–420
Onzo A, Hanna R, Zannou I, Sabelis MW, Yaninek JS (2003a) Dynamics of refuge use: diurnal, vertical migration by predatory and herbivorous mites within cassava plants. Oikos 101:59–69
Onzo A, Hanna R, Sabelis MW (2003b) Interactions in an acarine predator guild: Impact on Typhlodromalus aripo abundance and biological control of cassava green mite in Africa. Exp Appl Acarol 31:225–241
Onzo A, Hanna R, Janssen A, Sabelis MW (2004) Interactions between two neotropical phytoseiid predators on cassava plants and consequences for biological control of a shared spider mite prey—a screenhouse evaluation. Biocont Sci Tech 14:63–76
Onzo A, Hanna R, Negloh K, Toko M, Sabelis MW (2005) Biological control of cassava green mite with exotic and indigenous phytoseiid predators—effects of intraguild predation and supplementary food. Biol Control 33:143–152
Onzo A, Hanna R, Sabelis MW (2009) Within-plant migration of the predatory mite Typhlodromalus aripo from the apex to the leaves of cassava: response to day–night cycle, prey location and prey density. J Insect Behav 22:186–195
Onzo A, Hanna R, Sabelis MW (2012) The predatory mite Typhlodromalus aripo prefers green-mite induced plant odours from pubescent cassava varieties. Exp Appl Acarol 58:359–370
Polis GA, Myers CA, Holt RD (1989) The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu Rev Ecol Syst 20:297–330
Prasad RP, Snyder WE (2006) Diverse trait-mediated interactions in a multi-predator, multi-prey community. Ecology 87:1131–1137
Relyea RA (2003) How prey respond to combined predators: a review and an empirical test. Ecology 84:1827–1839
Rosenheim JA, Kaya HK, Ehler LE, Marois JJ, Jaffee BA (1995) Intraguild predation among biological control agents: theory and evidence. Biol Control 5:303–335
Sabelis MW (1992) Arthropod predators. In: Crawley MJ (ed) Natural enemies, the population biology of predators, parasites and diseases. Blackwell, Oxford, pp 225–264
SAS Institute (2008) Software version 9.2 (TSMO) Cary, North Carolina, USA
Sih A, Crowley P, McPeek M, Petranka J, Strohmeier K (1985) Predation, competition, and prey communities: a review of field experiments. Annu Rev Ecol Syst 16:269–311
Sih A, Englund G, Wooster D (1998) Emergent impacts of multiple predators on prey. Trends Ecol Evol 13:325–329
Snyder WE, Snyder GB, Finke DL, Straub CS (2006) Predator biodiversity strengthens herbivore suppression. Ecol Lett 9:789–796
Snyder GB, Finke DB, Snyder WE (2008) Predator biodiversity strengthens aphid suppression across single- and multiple-species prey communities. Biol Control 44:52–60
Sokal RR, Rohlf FJ (1981) Biometry, 2nd edn. Freeman and Company, New York
Soluk DA (1993) Multiple predator effects: predicting combined functional response of stream fish and invertebrate predators. Ecology 74:219–225
Soluk DA, Collins NC (1988) Synergistic interactions between fish and stoneflies: facilitation and interference among stream predators. Oikos 52:94–100
Stiling P, Cornelissen T (2005) What makes a successful biocontrol agent? A meta-analysis of biological control agent performance. Biol Control 34:236–246
Straub CS, Snyder WE (2006) Species identity dominates the relationship between predator biodiversity and herbivore suppression. Ecology 87:277–282
Straub CS, Snyder WE (2008) Increasing enemy biodiversity strengthens herbivore suppression on two plant species. Ecology 89:1605–1615
Symondson WOC, Sunderland KD, Greenstone MH (2002) Can generalist predators be effective biocontrol agents? Annu Rev Entomol 47:561–594
Tylianakis JM, Romo CM (2010) Natural enemy diversity and biological control: making sense of the context-dependency. Basic Appl Ecol 11:657–668
Yaninek JS, Hanna R (2003) Cassava green mite in Africa—a unique example of successful classical biological control of a mite pest on a continental scale. In: Neuenschwander P, Borgemeister C, Langewald L (eds) Biological control in IPM systems in Africa. CABI Publishing, Wallingford, pp 61–75
Yaninek JS, Onzo A (1988) Survey of cassava green mite in the People’s Republic of Benin, Jan 1988. IITA publication series. IITA, Ibadan, Nigeria. p 30
Yaninek JS, Saizonou S, Onzo A, Zannou I, Gnanvossou D (1996) Seasonal and habitat variability in the fungal pathogens, Neozygites cf. florida and Hirsutella. thompsonii, associated with cassava mites in Benin, West Africa. Biocont Sci Tech 1:323–330
Yaninek JS, Mégevand B, Ojo B, Cudjoe AR, Abole E, Onzo A, Zannou I (1998) Establishment and spread of Typhlodromalus manihoti (Acari; Phytoseiidae), an introduced phytoseiid predator of Mononychellus tanajoa (Acari: Tetranychidae) in Africa. Environ Entomol 276:1496–1505
Zannou ID, Hanna R, De Moraes GJ, Kreiter S (2005) Cannibalism and interspecific predation in a phytoseiid predator guild from cassava fields in Africa: evidence from the laboratory. Exp Appl Acarol 37:27–42
Zannou ID, Hanna R, Agboton B, De Moraes GJ, Kreiter S, Phiri G, Jone A (2007) Native phytoseiid mites as indicators of non-target effects of the introduction of Typhlodromalus aripo for the biological control of cassava green mite in Africa. Biol Control 41:190–198
Acknowledgments
We acknowledge the assistance of Sam Korie in statistical analysis and of Dr Ignace Zannou in identifying phytoseiids specimens. We are very grateful to B. Bovis, H. Dossounon, R. Houndafotché and M. Adandé for their technical assistance, both in field and in the laboratory. We are honored by the full cooperation of farmers in the Department of Mono who allowed us to conduct the experiments in their cassava fields. Three anonymous reviewers provided valuable comments on an earlier version of the manuscript. This research was supported with funds provided to the International Institute of Tropical Agriculture (IITA) by the International Fund for Agricultural Development (IFAD). In addition, a postdoctoral fellowship was granted to A. Onzo, based on funds provided to the University of Amsterdam and IITA by the Netherlands Foundation for the Advancement of Tropical Research (WOTRO).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Onzo, A., Sabelis, M.W. & Hanna, R. Single versus multiple enemies and the impact on biological control of spider mites in cassava fields in West-Africa. Exp Appl Acarol 62, 293–311 (2014). https://doi.org/10.1007/s10493-013-9742-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-013-9742-2