Abstract
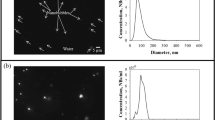
This paper investigates the stability of nitrogen nanobubbles under different concentrations of nitrogen molecules by molecular dynamics simulations. It is found that the stability of nanobubbles is very sensitive to the concentration of nitrogen molecules in water. A sharp transition between disperse states and assemble states of nitrogen molecules is observed when the concentration of nitrogen molecules is changed. The relevant critical concentration of nitrogen molecules needed by the existing nitrogen nanobubbles is analyzed.
Similar content being viewed by others
References
Parker, J. L., Claesson, P. M., and Attard, P. Bubbles, cavities, and the long-ranged attraction between hydrophobic surfaces. Journal of Physical Chemistry, 98(34), 8468–8480 (1994)
Neto, C., Evans, D. R., Bonaccurso, E., Butt, H. J., and Craig, V. S. J. Boundary slip in Newtonian liquids: a review of experimental studies. Reports on Progress in Physics, 68(12), 2859–2897 (2005)
De Gennes, P. G. On fluid/wall slippage. Langmuir, 18(9), 3413–3414 (2002)
Lou, S. T., Ouyang, Z. Q., Zhang, Y., Li, X. J., Hu, J., Li, M. Q., and Yang, F. J. Nanobubbles on solid surface imaged by atomic force microscopy. Journal of Vacuum Science & Technology B, 18(5), 2573–2575 (2000)
Zhang, X. H., Zhang, X. D., Lou, S. T., Zhang, Z. X., Sun, J. L., and Hu, J. Degassing and temperature effects on the formation of nanobubbles at the mica/water interface. Langmuir, 20(9), 3813–3815 (2004)
Zhang, X. H., Khan, A., and Ducker, W. A. A nanoscale gas state. Physical Review Letters, 98(13), 136101 (2007)
Tyrrell, J. W. G. and Attard, P. Images of nanobubbles on hydrophobic surfaces and their interactions. Physical Review Letters, 87(17), 176104 (2001)
Tyrrell, J. W. G. and Attard, P. Atomic force microscope images of nanobubbles on a hydrophobic surface and corresponding force-separation data. Langmuir, 18(1), 160–167 (2002)
Agrawal, A., Park, J., Ryu, D. Y., Hammond, P. T., Russell, T. P., and McKinley, G. H. Controlling the location and spatial extent of nanobubbles using hydrophobically nanopatterned surfaces. Nano Letters, 5(9), 1751–1756 (2005)
Zhang, X. H., Maeda, N., and Craig, V. S. J. Physical properties of nanobubbles on hydrophobic surfaces in water and aqueous solutions. Langmuir, 22(11), 5025–5035 (2006)
Zhang, X. H., Zhang, X., Sun, J., Zhang, Z., Li, G., Fang, H., Xiao, X., Zeng, X., and Hu, J. Detection of novel gaseous states at the highly oriented pyrolytic graphite-water interface. Langmuir, 23(4), 1778–1783 (2007)
Kikuchi, K., Ioka, A., Oku, T., Tanaka, Y., Saihara, Y., and Ogumi, Z. Concentration determination of oxygen nanobubbles in electrolyzed water. Journal of Colloid and Interface Science, 329(2), 306–309 (2009)
Ljunggren, S. and Eriksson, J. C. The lifetime of a colloid-sized gas bubble in water and the cause of the hydrophobic attraction. Colloids and Surfaces A-Physicochemical and Engineering Aspects, 130, 151–155 (1997)
Zhang, L., Chen, H., Li, Z., Fang, H., and Hu, J. Long lifetime of nanobubbles due to high inner density. Science in China Series G-Physics Mechanics & Astronomy, 51(2), 219–224 (2008)
Kameda, N., Sogoshi, N., and Nakabayashi, S. Nitrogen nanobubbles and butane nanodroplets at Si(100). Surface Science, 602(8), 1579–1584 (2008)
Ushikubo, F. Y., Furukawa, T., Nakagawa, R., Enari, M., Makino, Y., Kawagoe, Y., Shiina, T., and Oshita, S. Evidence of the existence and the stability of nano-bubbles in water. Colloids and Surfaces A-Physicochemical and Engineering Aspects, 361(1–3), 31–37 (2010)
Haebich, A., Ducker, W., Dunstan, D. E., and Zhang, X. H. Do stable nanobubbles exist in mixtures of organic solvents and water? Journal of Physical Chemistry B, 114(20), 6962–6967 (2010)
Holmberg, M., Kuhle, A., Garnaes, J., Morch, K. A., and Boisen, A. Nanobubble trouble on gold surfaces. Langmuir, 19(25), 10510–10513 (2003)
Tolman, R. C. The effect of droplet size on surface tension. Journal of Chemical Physics, 17(3), 333–337 (1949)
Zang, J. L. and Zhao, Y. P. A diffusion and curvature dependent surface elastic model with application to stress analysis of anode in lithium ion battery. International Journal of Engineering Science, 61 156–170 (2012)
Zhao, Y. P. Physical Mechanics of Surfaces and Interfaces (in Chinese), Science Press, Beijing, (2012)
Fang, H. and Hu, J. Molecular dynamics simulation studies on some topics of water molecules on hydrophobic surfaces. Nuclear Science and Techniques, 17(2), 71–77 (2006)
Wang, C. L., Li, Z. X., Li, J. Y., Xiu, P., Hu, J., and Fang, H. P. High density gas state at water/graphite interface studied by molecular dynamics simulation. Chinese Physics B, 17(7), 2646–2654 (2008)
Brenner, M. P. and Lohse, D. Dynamic equilibrium mechanism for surface nanobubble stabilization. Physical Review Letters, 101(21), 214505 (2008)
Hess, B., Kutzner, C., van der Spoel, D., and Lindahl, E. Gromacs 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. Journal of Chemical Theory and Computation, 4(3), 435–447 (2008)
Rappe, A. K., Casewit, C. J., Colwell, K. S., Goddard, W. A., and Skiff, W. M. UFF, a full periodic-table force-field for molecular mechanics and molecular-dynamics simulations. Journal of the American Chemical Society, 114(25), 10024–10035 (1992)
Ten Wolde, P. R. and Frenkel, D. Computer simulation study of gas-liquid nucleation in a lennardjones system. Journal of Chemical Physics, 109(22), 9901–9918 (1998)
Stillinger, F. H. Rigorous basis of Frenkel-Band theory of association equilibrium. Journal of Chemical Physics, 38(7), 1486–1494 (1963)
Nadassy, K., Wodak, S. J., and Janin, J. Structural features of protein-nucleic acid recognition sites. Biochemistry, 38(7), 1999–2017 (1999)
Yuan, Q. Z. and Zhao, Y. P. Precursor film in dynamic wetting, electrowetting, and electro-elastocapillarity. Physical Review Letters, 104(24), 246101 (2010)
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the National Natural Science Foundation of China (Nos. 10825520 and 11105088) and the Innovation Program of Shanghai Municipal Education Commission (No. 11YZ20)
Rights and permissions
About this article
Cite this article
Zhang, M., Tu, Ys. & Fang, Hp. Concentration of nitrogen molecules needed by nitrogen nanobubbles existing in bulk water. Appl. Math. Mech.-Engl. Ed. 34, 1433–1438 (2013). https://doi.org/10.1007/s10483-013-1757-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10483-013-1757-x