Abstract
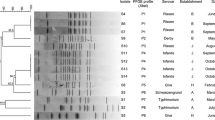
Hydrogen sulfide (H2S) detection is a screening method for distinguishing and identifying Salmonella strains from other bacteria in the intestine. Incidences of H2S-negative Salmonella have recently been reported in different countries. Although a high resistance rate against antimicrobial agents has been reported for H2S-positive Salmonella in many regions of the world, there is increasing evidence that high resistance to antibiotics has also increased in many H2S-negative Salmonella isolates. In this study, molecular characterisation of three H2S-negative Salmonella Havana, isolated from cloacal swab samples of broiler chickens, was performed. The phsA, phsB and phsC genes of the phs operon, which is responsible for hydrogen sulfide production, were amplified. Sequence analysis was then performed to identify mutations in the gene cluster. The antimicrobial resistance profiles of the isolates were determined by disc diffusion. Molecular characterisation was performed by multilocus sequence typing (MLST) and pulsed field gel electrophoresis (PFGE). The sequence analysis showed identified five point mutations in the phsA gene and one point mutation in the phsC gene in all isolates. The antibiotic resistance profile showed that the strains were resistant to cefoxitin and ceftazidime. MLST analysis showed that all strains belonged to sequence type (ST) 1621. This study is the first to report the H2S-negative S. Havana serotype.

Similar content being viewed by others
References
Albert MJ et al (2014) Isolation of salmonella enterica serovar kentucky strain ST 198 and its H2S-negative variant from a patient: implications for diagnosis. J Clin Microbiol 52:4090–4093. https://doi.org/10.1128/Jcm.01775-14
Asai T et al (2010) Molecular typing and antimicrobial resistance of Salmonella enterica subspecies enterica serovar Choleraesuis isolates from diseased pigs in Japan. Comput Immunol Microb 33:109–119. https://doi.org/10.1016/j.cimid.2008.08.004
Asgharpour F, Rajabnia R, Shahandashti EF, Marashi MA, Khalilian M, Moulana Z (2014) Investigation of class I integron in salmonella infantis and its association with drug resistance jundishapur. J Microb. https://doi.org/10.5812/jjm.10019
Barrett TJ, Gerner-Smidt P, Swaminathan B (2006) Interpretation of pulsed-field gel electrophoresis patterns in foodborne disease investigations and surveillance. Foodborne Pathog Dis 3:20–31. https://doi.org/10.1089/fpd.2006.3.20
Barrow PAJ, Thomson N (2010) Pathogenesis of bacterial infections in animals. 4th edn.
Barton LL, Fardeau ML, Fauque GD (2014) Hydrogen sulfide: a toxic gas produced by dissimilatory sulfate and sulfur reduction and consumed by microbial oxidation. Met Ions Life Sci 14:237–277. https://doi.org/10.1007/978-94-017-9269-1_10
Clark MA, Barrett EL (1987) The phs gene and hydrogen-sulfide production by salmonella-typhimurium. J Bacteriol 169:2391–2397. https://doi.org/10.1128/jb.169.6.2391-2397.1987
Fong CLW, Heinzinger NK, Tongklan S, Barrett EL (1993) Cloning of the phs genetic-locus from salmonella-typhimurium and a role for a phs product in its own induction. J Bacteriol 175:6368–6371
Heinzinger NK, Fujimoto SY, Clark MA, Moreno MS, Barrett EL (1995) Sequence analysis of the phs operon in Salmonella typhimurium and the contribution of thiosulfate reduction to anaerobic energy metabolism. J Bacteriol 177:2813–2820
Kariuki S, Onsare RS (2015) Epidemiology and genomics of invasive nontyphoidal salmonella infections in Kenya. Clin Infect Dis 61:S317–S324. https://doi.org/10.1093/cid/civ711
Kuo HC et al (2014) An association of genotypes and antimicrobial resistance patterns among salmonella isolates from pigs and humans in Taiwan. Plos One. https://doi.org/10.1371/journal.pone.0095772
Kwambana-Adams B et al (2015) Salmonella infections in the Gambia, 2005–2015. Clin Infect Dis 61:S354–S362. https://doi.org/10.1093/cid/civ781
Levitt MD, Furne J, Springfield J, Suarez F, DeMaster E (1999) Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. J Clin Invest 104:1107–1114. https://doi.org/10.1172/JCI7712
Li Y et al (2014) Nontyphoidal salmonella infection in children with acute gastroenteritis: prevalence, serotypes, and antimicrobial resistance in Shanghai. China Foodborne Pathog Dis 11:200–206. https://doi.org/10.1089/fpd.2013.1629
Lin D, Yan M, Lin S, Chen S (2014) Increasing prevalence of hydrogen sulfide negative Salmonella in retail meats. Food Microbiol 43:1–4. https://doi.org/10.1016/j.fm.2014.04.010
Majowicz SE et al (2010) The global burden of nontyphoidal salmonella gastroenteritis. Clin Infect Dis 50:882–889. https://doi.org/10.1086/650733
Majtanova L, Majtan T, Majtan V (2010) Detection of the class 1 integrons and SGI1 among Salmonella enterica Serovar Typhimurium DT104, U302, DT120, DT193, and nontypable human isolates. Jpn J Infect Dis 63:292–295
Ruby T, McLaughlin L, Gopinath S, Monack D (2012) Salmonella's long-term relationship with its host Fems. Microbiol Rev 36:600–615. https://doi.org/10.1111/j.1574-6976.2012.00332.x
Sakano C et al (2013) Genetic analysis of non-hydrogen sulfide-producing Salmonella enterica serovar typhimurium and S. enterica serovar infantis isolates in Japan. J Clin Microbiol 51:328–330. https://doi.org/10.1128/JCM.02225-12
Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239
Thiennimitr PWS, Winter MG, Xavier MN, Tolstikov V, Huseby DL, Sterzenbach T, Tsolis RM, Roth JR, Baumler AJ (2011) Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. PNAS 108:17480–17485. https://doi.org/10.1073/pnas.1107857108
Winter SE et al (2010) Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467:426–429. https://doi.org/10.1038/nature09415
Wu FL et al (2016) Molecular characterization of salmonella enterica serovar aberdeen negative for H2S production in China. Plos One. https://doi.org/10.1371/journal.pone.0161352
Xie J et al (2018) Antibiotic resistance and molecular characterization of the hydrogen sulfide-negative phenotype among diverse Salmonella serovars in China. Bmc Infect Dis. https://doi.org/10.1186/s12879-018-3209-3
Xie J et al (2015) Antimicrobial resistance and molecular investigation of H2S-negative salmonella enterica subsp enterica serovar choleraesuis isolates in China. Plos One. https://doi.org/10.1371/journal.pone.0139115
Yi S et al (2014) Emergence and prevalence of non-H2S-producing Salmonella enterica serovar Senftenberg isolates belonging to novel sequence type 1751 in China. J Clin Microbiol 52:2557–2565. https://doi.org/10.1128/JCM.00377-14
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Müştak, İ.B., Müştak, H.K. & Sarıçam, S. Molecular characterisation of hydrogen sulfide negative Salmonella enterica serovar Havana. Antonie van Leeuwenhoek 113, 1241–1246 (2020). https://doi.org/10.1007/s10482-020-01432-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-020-01432-3