Abstract
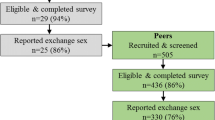
Traditional recruitment methods for microbicide efficacy trials are labor intensive and may fail to reach high-risk hard-to-reach populations. We report duration of recruitment and lessons learned from a two-stage process to recruit female sex workers (FSWs) into a placebo microbicide trial, and examined characteristics associated with successful recruitment of peers who screened for and enrolled in the trial. FSWs were first recruited via respondent-driven sampling (RDS) to complete a survey and subsequently invited to screen for enrollment into a placebo microbicide trial taking place at a local clinic. It took 6 months to enroll 267 participants into the trial. Successful recruiters of peers who enrolled were more likely to have enrolled themselves (AOR 2.0, CI 1.3–2.9) and less likely to visit Nellore city (AOR 0.5, CI 0.3–0.9). Recruitment of FSWs via a two-stage recruitment strategy with RDS can be a good option for future clinical trials.

Similar content being viewed by others
References
National AIDS Control Organization, Department of AIDS Control, Ministry of Health and Family Welfare, Government of India. Annual Report 2012–2013. New Delhi, India 2013.
Joshi S, Solomon S, Mayer K, Mehendale S. Preparing for efficacy trials of vaginal microbicides in Indian women. Indian J Med Res. 2005;121(4):502–9.
National AIDS Control Organization, Ministry of Health and Family Welfare, Government of India. Annual Report 2010–2011. New Delhi 2011.
Toerien M, Brookes ST, Metcalfe C, et al. A review of reporting of participant recruitment and retention in RCTs in six major journals. Trials. 2009;10:52.
Campbell MK, Snowdon C, Francis D, et al. Recruitment to randomised trials: strategies for trial enrollment and participation study. The STEPS study. Health Technol Assess. 2007;11(48):iii–ix.
Guest G, Severy L, von Mollendorf C, Van Damme L. Overcoming recruitment challenges: lessons learned from a safety and feasibility study of a diaphragm/microbicide combination in South Africa. J Acquir Immune Defic Syndr. 2007;45(4):481–2.
Chatterjee P. AIDS in India: police powers and public health. The Lancet. 2006;367:805–6.
Biradavolu MR, Burris S, George A, Jena A, Blankenship KM. Can sex workers regulate police? Learning from an HIV prevention project for sex workers in southern India. Soc Sci Med. 2009;68(8):1541–7.
Heckathorn D. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl. 1997;44(2):174–99.
Scott G. “They got their program, and I got mine”: a cautionary tale concerning the ethical implications of using respondent-driven sampling to study injection drug users. Int J Drug Policy. 2008;19(1):42–51.
Heckathorn D, Semaan S, Broadhead RS, Hughes J. Extensions of respondent-driven sampling: a new approach to the study of injection drug users aged 18–25. AIDS Behav. 2002;6(1):55–67.
Magnani R, Sabin K, Saidel T, Heckathorn D. Review of sampling hard-to-reach and hidden populations for HIV surveillance. Aids. 2005;19(2):S67–72.
Erausquin JT, Reed E, Blankenship KM. Police-related experiences and HIV risk among female sex workers in Andhra Pradesh, India. J Infect Dis. 2011;204(5):S1223–8.
Johnston LG, Sabin K, Mai TH, Pham TH. Assessment of respondent driven sampling for recruiting female sex workers in two Vietnamese cities: reaching the unseen sex worker. J Urban Health. 2006;83(6 Suppl):i16–28.
Wattana W, van Griensven F, Rhucharoenpornpanich O, et al. Respondent-driven sampling to assess characteristics and estimate the number of injection drug users in Bangkok, Thailand. Drug Alcohol Depend. 2007;90(2–3):228–33.
Yeka W, Maibani-Michie G, Prybylski D, Colby D. Application of respondent driven sampling to collect baseline data on FSWs and MSM for HIV risk reduction interventions in two urban centres in Papua New Guinea. J Urban Health. 2006;83(6):i60–72.
Johnston LG, Sabin K. Sampling hard-to-reach populations with respondent driven sampling. Methodological Innovations Online. 2010;5(2):38–48.
Kendall C, Kerr LRFS, Gondim RC, et al. An empirical comparison of respondent-driven sampling, time location sampling, and snowball sampling for behavioral surveillance in men who have sex with men, Fortaleza, Brazil. AIDS Behav. 2008;12:S97–104.
Malekinejad M, Johnston LG, Kendall C, Kerr LR, Rifkin MR, Rutherford GW. Using respondent-driven sampling methodology for HIV biological and behavioral surveillance in international settings: a systematic review. AIDS Behav. 2008;12(4):S105–30.
Jenkins R. Recruiting substance-using men who have sex with men into hiv prevention research: current status and future directions. AIDS Behav. 2012;16(6):1411–9.
Medhi GK, Mahanta J, Paranjape RS, Adhikary R, Laskar N, Ngully P. Factors associated with HIV among female sex workers in a high HIV prevalent state of India. AIDS Care. 2012;24(3):369–76.
Abbott SA, Friedland BA, Sarna A, et al. An evaluation of methods to improve the reporting of adherence in a placebo gel trial in Andhra Pradesh, India. AIDS Behav. 2013;17(6):2222–36.
Mensch BS, Friedland BA, Abbott SA, et al. Characteristics of female sex workers in southern india willing and unwilling to participate in a placebo gel trial. AIDS Behav. 2013;17(2):585–97.
Parivartan P. Results of a Cross-sectional Survey of Female Sex Workers in Rajahmundry, Andhra Pradesh. A Summary Report.: Yale University, CARE India, Avahan; May 2007.
Dandona R, Dandona L, Gutierrez JP, et al. High risk of HIV in non-brothel based female sex workers in India. BMC Public Health. 2005;5(87): Available from: http://www.biomedcentral.com [Accessed October 2007].
Sarna A, Friedland BA, Srikrishnan AK, et al. Sexually transmitted infections and reproductive health morbidity in a cohort of female sex workers screened for a microbicide feasibility study in Nellore, India. Glob J Health Sci. 2013;5(3):139–49.
Barroso PF, de Souza MB, do Lago RF. Barriers to recruit female commercial sex workers for HIV vaccine trials: the Rio de Janeiro experience. J Acquir Immune Defic Syndr. 2009;50(1):116–7.
Dutta S, Joglekar N, Joshi S, Steven JR. Experience of conducting a phase I safety & acceptability clinical trial of a candidate vaginal microbicide & lessons learned. Indian J Med Res. 2008;128(2):212–3.
Joglekar N, Joshi S, Kakde M, et al. Acceptability of PRO2000 vaginal gel among HIV un-infected women in Pune, India. AIDS Care. 2007;19(6):817–21.
Joglekar NS, Joshi SN, Navlakha SN, Katti UR, Mehendale SM. Acceptability of Praneem polyherbal vaginal tablet among HIV uninfected women & their male partners in Pune, India-Phase I study. Indian J Med Res. 2006;123(4):547–52.
Joglekar NS, Joshi SN, Deshpande SS, Parkhe AN, Katti UR, Mehendale SM. Acceptability and adherence: findings from a Phase II study of a candidate vaginal microbicide, ‘Praneem polyherbal tablet’, in Pune, India. Trans R Soc Trop Med Hyg. 2010;104(6):412–5.
Joshi S, Joglekar N, Ghate M, et al. Phase I safety & preliminary acceptability of nonoxynol-9 vaginal pessary as a vaginal microbicide in low risk women in Pune, India. Indian J Med Res. 2003;117:152–7.
Joshi SN, Katti U, Godbole S, et al. Phase I safety study of Praneem polyherbal vaginal tablet use among HIV-uninfected women in Pune, India. Trans R Soc Trop Med Hyg. 2005;99(10):769–74.
Mauck C, Joshi S, Schwartz J, Callahan M, Walsh T. Reddy female condom: functional performance of a 90-mm shaft length in two clinical studies. Contraception. 2011;83(5):466–71.
Mehendale S, Deshpande S, Kohli R, Tsui S, Tolley E. Acceptability of coitally-associated versus daily use of 1% tenofovir vaginal gel among women in Pune, India. Int Health. 2012;4:63–9.
Solomon SS, Solomon S, Masse BR, et al. Risk reduction counseling is associated with decreased hiv transmission-associated behaviors in high-risk indian heterosexuals. JAIDS J Acquir Immune Defic Syndr. 2006;42(4):478–83.
Van Damme L, Chandeying V, Ramjee G, et al. Safety of multiple daily applications of COL-1492, a nonoxynol-9 vaginal gel, among female sex workers. COL-1492 Phase II Study Group. AIDS. 2000;14(1):85–8.
Van Damme L, Govinden R, Mirembe FM, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359(5):463–72.
Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360(9338):971–7.
van De Wijgert J, Fullem A, Kelly C, et al. Phase 1 trial of the topical microbicide BufferGel: safety results from four international sites. J Acquir Immune Defic Syndr. 2001;26(1):21–7.
Mehendale SM, Ghate MV, Kishore Kumar B, et al. Low HIV-1 Incidence Among Married Serodiscordant Couples in Pune, India. JAIDS J Acquir Immune Defic Syndr. 2006;41(3):371–3. doi:10.1097/1001.qai.0000209905.0000235620.0000209948.
Kanouse D, Bluthenthal R, Bogart L, Iguchi M, Perry S, Sand K. Recruiting drug-using men who have sex with men into behavioral interventions: a two-stage approach. J Urban Health. 2005;82:i109–19.
Grov C, Bux D Jr, Parsons JT, Morgenstern J. Recruiting hard-to-reach drug-using men who have sex with men into an intervention study: lessons learned and implications for applied research. Subst Use Misuse. 2009;44(13):1855–71.
Morrow KM, Fava JL, Rosen RK, Christensen AL, Vargas S, Barroso C. Willingness to use microbicides varies by race/ethnicity, experience with prevention products, and partner type. Health Psychol. 2007;26(6):777–86.
Broadhead R, Heckathorn D, Weakliem D, et al. Harnessing peer networks as an instrument for AIDS prevention: results from a peer-driven intervention. Public Health Rep. 1998;113(1):42–57.
Broadhead R, Volkanevsky V, Rydanovab T, et al. Peer-driven HIV interventions for drug injectors in Russia: first year impact results of a field experiment. Int J Drug Policy. 2006;17:379–92.
Broadhead R, Heckathorn D, Grund J, Stern L, Anthony D. Drug users versus outreach workers in combating AIDS: preliminary results of a peer-driven intervention. J Drug Issues. 1995;25(3):531–64.
Broadhead R, Heckathorn D, Altice F, et al. Increasing drug users’ adherence to HIV treatment: results of a peer-driven intervention feasibility study. Soc Sci Med. 2002;55(2):235–46.
Gross M, Buchbinder SP, Celum C, Heagerty P, Seage GR III. Rectal microbicides for U.S. gay men: Are clinical trials needed? Are they feasible? Sex Transm Dis. 1998;25(6):296–302.
Tharawan K, Manopaiboon C, Ellertson C, et al. Women’s willingness to participate in microbide trials in northern Thailand. J Acquir Immune Defic Syndr Human Retrovirol. 2001;28(2):180–6.
van de Wijgert J, Coetzee N, de Kock A, Blanchard K, Jones H. Assessing selection bias in a microbicide trial. Paper presented at: Poster presentation at the International Congress of Sexually Transmitted Infections—ISSTDR/IUSTI, June 2001.
Acknowledgments
The authors would like to thank Ulrike Rawiel, as well as all YRG CARE study staff, particularly: K.R. Hari, Rajeswari and outreach volunteers; Durga, Jayanthi, Issaiah Kumari, Sowjanya, Sarojini, Prema Joythi, Dr. C.S. Shalini, Vijaya Lakshmi, K. Aparna, and Sasi J., for their dedicated work on this project. This manuscript is made possible by the generous support of the American and Indian people through the United States Agency for International Development (USAID), Bureau for Global Health, Office of Population and Reproductive Health, under the terms of Award No. GPO-A-00-04-00019; the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), under the terms of Award No. R21 HD060270; and the Indian Council of Medical Research (ICMR), under the terms of Award No. Indo-US/54/2007-ECD II. The opinions expressed herein are those of the author(s) and do not necessarily reflect the views of the U.S. or Indian governments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tun, W., Katzen, L.L., Abbott, S.A. et al. Using a 2-Stage Strategy with Respondent-Driven Sampling to Recruit a Hard-to-Reach Population for a Placebo Microbicide Gel Clinical Trial in Nellore, Andhra Pradesh (India). AIDS Behav 19, 369–379 (2015). https://doi.org/10.1007/s10461-014-0938-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-014-0938-1