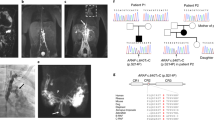
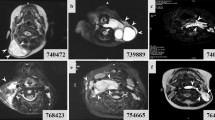
Abstract
Generalized lymphatic anomaly (GLA or lymphangiomatosis) is a rare disease characterized by a diffuse proliferation of lymphatic vessels in skin and internal organs. It often leads to progressive respiratory failure and death, but its etiology is unknown. Here, we isolated lymphangiomatosis endothelial cells from GLA tissue. These cells were characterized by high proliferation and survival rates, but displayed impaired capacities for migration and tube formation. We employed whole exome sequencing to search for disease-causing genes and identified a somatic mutation in NRAS. We used mouse and zebrafish model systems to initially evaluate the role of this mutation in the development of the lymphatic system, and we studied the effect of drugs blocking the downstream effectors, mTOR and ERK, on this disease.






Similar content being viewed by others
Abbreviations
- LEC:
-
Lymphatic endothelial cells
- HDLEC:
-
Human dermal lymphatic endothelial cells
- LyECs:
-
Lymphangiomatosis endothelial cells
- EC:
-
Endothelial clls
- MTOR:
-
Mammalian target of rapamycin
- VEGF-A:
-
Vascular endothelial growth factor A
- WES:
-
Whole exome sequencing
References
Wassef M, Blei F, Adams D et al (2015) Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics 136:e203–e214
Faul JL, Berry GJ, Colby TV et al (2000) Thoracic lymphangiomas, lymphangiectasis, lymphangiomatosis, and lymphatic dysplasia syndrome. Am J Respir Crit Care Med 161:1037–1046
Kransdorf MJ (1995) Benign soft-tissue tumors in a large referral population: distribution of specific diagnoses by age, sex, and location. AJR Am J Roentgenol 164:395–402
Kadakia KC, Patel SM, Yi ES, Limper AH (2013) Diffuse pulmonary lymphangiomatosis. Can Resp J J Can Thorac Soc 20:52–54
Trenor CC 3rd, Chaudry G (2014) Complex lymphatic anomalies. Semin Pediatr Surg 23:186–190
Hilliard RI, McKendry JB, Phillips MJ (1990) Congenital abnormalities of the lymphatic system: a new clinical classification. Pediatrics 86:988–994
Ozeki M, Fukao T, Kondo N (2011) Propranolol for intractable diffuse lymphangiomatosis. N Engl J Med 364:1380–1382
Bassi A, Syed S (2014) Multifocal infiltrative lymphangiomatosis in a child and successful treatment with sirolimus. Mayo Clin Proc 89:e129
Reinglas J, Ramphal R, Bromwich M (2011) The successful management of diffuse lymphangiomatosis using sirolimus: a case report. Laryngoscope 121:1851–1854
van Beijnum JR, Rousch M, Castermans K, van der Linden E, Griffioen AW (2008) Isolation of endothelial cells from fresh tissues. Nat Protoc 3:1085–1091
DeCicco-Skinner KL, Henry GH, Cataisson C et al (2014) Endothelial cell tube formation assay for the in vitro study of angiogenesis. J Vis Exp 91:e51312
Nicenboim J, Malkinson G, Lupo T et al (2015) Lymphatic vessels arise from specialized angioblasts within a venous niche. Nature 522:56–61
Avraham-Davidi I, Ely Y, Pham VN et al (2012) ApoB-containing lipoproteins regulate angiogenesis by modulating expression of VEGF receptor 1. Nat Med 18:967–973
Okuda KS, Astin JW, Misa JP, Flores MV, Crosier KE, Crosier PS (2012) lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development 139:2381–2391
Villefranc JA, Amigo J, Lawson ND (2007) Gateway compatible vectors for analysis of gene function in the zebrafish. Dev Dyn 236:3077–3087
Greenberger S, Boscolo E, Adini I, Mulliken JB, Bischoff J (2010) Corticosteroid suppression of VEGF-A in infantile hemangioma-derived stem cells. N Engl J Med 362:1005–1013
Greenberger S, Yuan S, Walsh LA, Boscolo E, Kang KT, Matthews B, Mulliken JB, Bischoff J (2011) Rapamycin suppresses self-renewal and vasculogenic potential of stem cells isolated from infantile hemangioma. J Invest Dermatol 131:2467–2476
Melero-Martin JM, De Obaldia ME, Kang SY et al (2008) Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res 103:194–202
Tamay Z, Saribeyoglu E, Ones U et al (2005) Diffuse thoracic lymphangiomatosis with disseminated intravascular coagulation in a child. J Pediatr Hematol Oncol 27:685–687
Satria MN, Pacheco-Rodriguez G, Moss J (2011) Pulmonary lymphangiomatosis. Lymphat Res Biol 9:191–193
Jurisic G, Detmar M (2009) Lymphatic endothelium in health and disease. Cell Tissue Res 335:97–108
Tammela T, Alitalo K (2010) Lymphangiogenesis: molecular mechanisms and future promise. Cell 140:460–476
Li A, Ma Y, Jin M et al (2012) Activated mutant NRas(Q61 K) drives aberrant melanocyte signaling, survival, and invasiveness via a Rac1-dependent mechanism. J Investig Dermatol 132:2610–2621
Mor A, Philips MR (2006) Compartmentalized Ras/MAPK signaling. Annu Rev Immunol 24:771–800
Mandala M, Merelli B, Massi D (2014) Nras in melanoma: targeting the undruggable target. Crit Rev Oncol/Hematol 92:107–122
Hammill AM, Wentzel M, Gupta A et al (2011) Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer 57:1018–1024
Lackner H, Karastaneva A, Schwinger W et al (2015) Sirolimus for the treatment of children with various complicated vascular anomalies. Eur J Pediatr 174:1579–1584
Adams DM, Trenor CC, Hammill AM et al (2016) Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics 137(2):e20153257
Grunewald TG, Damke L, Maschan M et al (2010) First report of effective and feasible treatment of multifocal lymphangiomatosis (Gorham-Stout) with bevacizumab in a child. Ann Oncol 21:1733–1734
Aman J, Thunnissen E, Paul MA, van Nieuw Amerongen GP, Vonk-Noordegraaf A (2012) Successful treatment of diffuse pulmonary lymphangiomatosis with bevacizumab. Ann Intern Med 156:839–840
Rockson SG (2014) Laboratory models for the investigation of lymphangiomatosis. Microvasc Res 96:64–67
Khan ZA, Boscolo E, Picard A et al (2008) Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Investig 118:2592–2599
Boscolo E, Limaye N, Huang L et al (2015) Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. J Clin Investig 125:3491–3504
Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM (2003) Angiogenic network formation in the developing vertebrate trunk. Development 130:5281–5290
Cirstea IC, Kutsche K, Dvorsky R et al (2010) A restricted spectrum of NRAS mutations causes Noonan syndrome. Nat Genet 42:27–29
Lanning P, Simila S, Suramo I, Paavilainen T (1978) Lymphatic abnormalities in Noonan’s syndrome. Pediatr Radiol 7:106–109
Burrows PE, Gonzalez-Garay ML, Rasmussen JC et al (2013) Lymphatic abnormalities are associated with RASA1 gene mutations in mouse and man. Proc Natl Acad Sci USA 110:8621–8626
Lapinski PE, Kwon S, Lubeck BA et al (2012) RASA1 maintains the lymphatic vasculature in a quiescent functional state in mice. J Clin Investig 122:733–747
Chapman PB, Solit DB, Rosen N (2014) Combination of RAF and MEK inhibition for the treatment of BRAF-mutated melanoma: feedback is not encouraged. Cancer Cell 26:603–604
Atefi M, Titz B, Avramis E et al (2015) Combination of Pan-RAF and MEK inhibitors in NRAS mutant melanoma. Mol Cancer 14:27
Casanova JL, Conley ME, Seligman SJ, Abel L, Notarangelo LD (2014) Guidelines for genetic studies in single patients: lessons from primary immunodeficiencies. J Exp Med 211:2137–2149
Buhrman G, Holzapfel G, Fetics S, Mattos C (2010) Allosteric modulation of Ras positions Q61 for a direct role in catalysis. Proc Natl Acad Sci USA 107:4931–4936
Malumbres M, Barbacid M (2003) RAS oncogenes: the first 30 years. Nat Rev Cancer 3:459–465
Lokmic Z, Mitchell GM, Koh Wee Chong N, Bastiaanse J, Gerrand YW, Zeng Y, Williams ED, Penington AJ (2014) Isolation of human lymphatic malformation endothelial cells, their in vitro characterization and in vivo survival in a mouse xenograft model. Angiogenesis 17(1):1–15. https://doi.org/10.1007/s10456-013-9371-8
Acknowledgements
We thank M. Grunspan for help with zebrafish experiments; Sarit Farage-Barhom for her help with bioinformatics analysis; Michael Dellinger, The Lymphatic Malformation Institute (LMI) for fruitful discussions. This project was supported by grants from the Israel Science Foundation (Grant No. 1716/11 to S.G.), Basil O’Connor Starter Scholar Research Award Grant from the March of Dimes (No. 5-FY12-55, to S.G), Marie Curie Re-integration grant (FP7-PEOPLE-2010-IRG to S.G).
Author information
Authors and Affiliations
Contributions
E.M., G.L., N.A., A.B.,G.R., K.Y., and S.G. designed research studies; E.M., G.L., O.B., I.D., L.S., E.E., A.H., and K.C. conducted experiments and acquired data; E.M., G.L., O.B., I.D., L.S., E.E., I.P., and U.R., A.B., G.R., K.Y., and S.G analyzed and interpreted data; I.P, and U.R. provided human tissue samples; E.M., G.L., G.R., K.Y., and S.G wrote the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Manevitz-Mendelson, E., Leichner, G.S., Barel, O. et al. Somatic NRAS mutation in patient with generalized lymphatic anomaly. Angiogenesis 21, 287–298 (2018). https://doi.org/10.1007/s10456-018-9595-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-018-9595-8