Abstract
The exposure to airborne dusts and microorganisms is associated with respiratory diseases and other health effects. Currently, research concerning fungal aerosol and particulate matter levels in animal enclosures of zoological gardens is very scarce. Such premises are characterized by numerous sources of air contamination, and they are both working environment and popular tourist attractions. This study aimed to assess the levels of fungal aerosol in animal premises (tapirs, giraffes, pygmy hippos, monkeys, meerkats, pheasants, ostriches, exotarium) of the Kraków Zoo. The bioaerosol samples were collected using a six-stage Andersen impactor to assess the concentrations and size distribution of airborne fungi. Particulate matter (PM10, PM4, PM2.5 and PM1) concentration was assessed using DustTrak Aerosol Monitor. PM concentrations were relatively low (0.048–0.204 mg/m3) and did not exceed the limit values for occupational exposure. Also, the levels of fungal aerosol in almost all cases did not exceed the limit values. The smallest concentration of the total fungal aerosol was observed in the rooms for hippos (approx. 970 CFU/m3) while the highest—in exotarium (16,800 CFU/m3). The share of respirable fraction of bioaerosol was quite high—from 62.6% in monkey houses to 89.2% in exotarium. Having in mind that bioaerosol-forming fungi may contain allergenic species, attention should be paid to thorough cleaning of animals and their premises, and maintaining appropriate levels of microclimatic parameters in the rooms.
Similar content being viewed by others
1 Introduction
Animals in zoological gardens are entirely dependent on the conditions created by people. Some animals can use space outside their buildings in the form of a run, aviary or similar objects. Rooms, in which appropriate conditions for their well-being (or in other words animal welfare) are ensured, are necessary—for some animals only in the cold season, while for others—throughout the year. Animal welfare includes appropriately selected room parameters: adequate surface and cubature, sufficient amount of light, as well as adequate temperature and humidity (Pawlak et al. 2008). Ensuring animal welfare in a zoo is a requirement of the EU Council Directive 1999/22/EC and a prerequisite for its operation. It means meeting the so-called five freedoms, i.e., animal freedom from hunger and thirst, pain, injuries and illness, discomfort, fear and stress, and unnatural behavior caused by lack of space (Webster 1994).
In order to avoid diseases of animals kept in zoos, which may be associated with the simultaneous occurrence of discomfort and stress, proper hygiene conditions need to be ensured. Regardless of how good indoor conditions animals are guaranteed, their mere presence in the rooms in connection with microorganisms dwelling on them, as well as the presence of litter, feces and food residues combined with optimal temperature and humidity, favors the growth and proliferation of molds. Their spores float in the air leading to microbial contamination. The occurrence of airborne molds depends to the greatest extent on the species of animals inhabiting the rooms, stocking density, health status of animals, type of food and microclimatic parameters prevailing in the rooms. The type and degree of mycological contamination of the air in animal premises are an indicator of sanitary and hygienic condition therein (Matković et al. 2009; Budzińska et al. 2014). However, the previous studies showed that animal premises of different types are characterized by relatively high concentrations of different biological agents, including microbial aerosol, usually transported by organic dust particles present in animal rooms (Sowiak et al. 2012). Fungal aerosols produced in animal houses may pose threat to animals and their caretakers. Also, by the spread of bioaerosol outside the buildings, they may cause local or even more extensive contamination of the external environment. The level of environmental contamination depends on the microbial count, their potential pathogenicity and biological viability, as well as on the current topographic and atmospheric characteristics of the environment (Matković et al. 2007). Mycological pollution of both indoor and outdoor air is correlated with the concentration of dust in the air of buildings. Airborne fungi and their spores form complexes with dust particles, the composition of which greatly facilitates their growth rate and survival in unfavorable environmental conditions. The excessive mycological contamination of air can lead to adverse health effects in both animals and people who work with them. The most common health issues in such cases include: asthma, allergic alveolitis or toxic organic dust syndrome (Kołacz and Dobrzański 2006; Millner 2009; Samadi et al. 2013; Butler and Madhavan 2017; Douglas et al. 2018). It has been demonstrated that inhalation of fungal aerosols can affect humans in three major ways: by causing infectious diseases, by inducing and enhancing allergic reactions and by causing mycotoxin-induced severe toxic reactions, sometimes leading to death in immunocompromised people (Priyamvada et al. 2017).
Another important aspect of the potential health effects of bioaerosol and airborne dust particles is related to their aerodynamic diameter, which affects the penetrability to the human respiratory system (Sowiak et al. 2012). Particles of aerodynamic diameter exceeding 5 µm deposit mainly in the nasal and pharyngeal zone, but those whose diameters are lower than 5 µm (called the respirable fraction) reach as far as the alveoli, causing more severe allergenic or toxic effects (Owen et al. 1992).
There have been numerous studies on microbial aerosol in animal production premises (e.g., Dutkiewicz et al. 1994; Jacobson et al. 2003; Ławniczek-Wałczyk et al. 2013; Budzińska et al. 2014; Matković et al. 2007, 2009; Millner 2009; Sowiak et al. 2012; Viegas et al. 2013) or in farming environment (e.g., Karwowska 2005; Douglas et al. 2018), but none of those referred to non-production facilities, such as zoological gardens. In a previous study, Grzyb and Lenart-Boroń (2019) investigated the concentration and size distribution of bacterial aerosol in some premises of a Zoological Garden in Kraków and indicated that animals can be a significant source of bacterial bioaerosol components, but the levels of bioaerosol may vary significantly depending on the animals kept in the premises. Moreover, the respirable fraction of bioaerosol was predominant, reaching 70% of the total fraction in some cases, which indicates possible health threats to the people exposed. This is an important fact, given the specificity of zoological gardens, as they are both working environments and are among the most popular tourist objects visited by families with small children. Also, the world literature lacks reports on the mycological surveys with the consideration of particle diameters in connection with the determination of airborne dust concentration and microclimate parameters in animal premises in zoos. The current study was aimed to fill this gap. The results obtained in the course of the study are expected to give background for assessing the potential exposure of both visitors and workers of the zoo to airborne dust and fungi, as well as to determine whether microclimatic factors or dust particle size affect the concentration of airborne fungi with various particle sizes.
2 Materials and methods
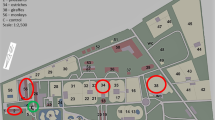
The study was conducted in a Zoological Garden in Kraków in three seasons: autumn, winter and spring (from October 2016 to May 2017). Summer season was not included in the analysis, as the animals spend most of their time outdoors during this season. The measurements were conducted in four replicates, and the samples were collected inside the premises where animals are kept. The animals included in this study were: five species of mammals (tapirs, giraffes, pygmy hippos, monkeys and meerkats) and two species of birds (pheasants and ostriches), as well as amphibia, reptiles and fish (in their room, called exotarium). The selection of animals for this study was based on the fact that those were the only groups that use shelters in the Kraków Zoo in the study period. One site, not inhabited by animals, located outdoors at a distance of approx. 10 m from one of the analyzed buildings was treated as control. Location of the sampling sites is shown in Fig. 1, and their characteristics are shown in Table 1. Each time, the collection of samples was conducted between 7 and 9 a.m., i.e., before the opening of the Zoological Garden.
The air samples were collected using a six-stage Andersen–Graseby cascade impactor, model WES-710 (Westech Instrument, UK), the use of which allows to distinguish the following aerodynamic diameters of bioaerosol: 7 µm (stage one, F1), 4.7–7 µm (stage two, F2), 3.3–4.7 µm (stage three, F3), 2.1–3.3 µm (stage four, F4), 1.1–2.1 µm (stage five, F5) and 0.65–1.1 µm (stage six, F6). The sampling time was 1–4 min, depending on the expected concentration of airborne microorganisms, which was pre-assessed based on the dust concentration. Therefore, having the air flow through the impactor of 28.3 dm3/min, the volume of aspirated air ranged from 28.3 to 113.2 dm3. Each sample included six impaction stages—given that the analyses were conducted in four replicates, in nine sites (eight premises for animals and one control site), 216 samples were collected in each season; in total, 648 Petri plates with biological material were analyzed during the study period. The impactor was placed at the height of 1.5 m above the ground level to collect the air from the human breathing zone. Malt Extract Agar (Biocorp, Poland) was used for the cultivation of molds and yeasts. The samples were incubated at 30 °C for 4 days, followed by 4 days at 22 °C in aerobic conditions. The prolonged incubation of samples enables the growth of slowly growing strains at a lower temperature range. After incubation, the typical fungal colonies were counted, and the results were expressed as the number of colony forming units per m3 of air (CFU/m3).
The particulate matter concentration was measured using a DustTrak™ II Aerosol Monitor 8530 (TSI Inc., USA) laser photometer. The device allows to measure four fractions of dust: PM10 (i.e., dust particles not larger than 10 μm), PM4, PM2.5 and PM1 (dust particles with diameters below 4, 2.5 and 1 μm, respectively), using interchangeable heads. The sampling time for each dust fraction was 1 min, during which the device noted 20 results, as the sampling was set every 3 s.
Microclimatic parameters (temperature and relative humidity) were measured using the Kestrel 4000 Weather Meter (Nielsen-Kellerman, USA).
Aggregates containing microorganisms with aerodynamic diameters below 4.7 µm were treated as the respirable fraction (RF) of bioaerosol. In the case of dust, it was assumed that the respirable fraction consists of dust particles smaller than 4.0 µm.
The recorded bioaerosol concentrations, due to the absence of guidelines on the acceptable concentrations of microorganisms in animal rooms, were referred to the proposal of the Team of Experts in Biological Factors (Pol.: ZECB) (Augustyńska and Pośniak 2016) on the recommended concentrations of airborne microorganisms, treating animal rooms as organic dust-contaminated working premises, which is 50,000 CFU/m3 for the total count and 25,000 CFU/m3 for the respirable fraction of fungi.
Statistical analysis was performed using the Statistica v. 13 software (StatSoft, USA). The observed values of fungal aerosol are presented as medians and ranges. The normality of data distribution was tested using the Shapiro–Wilk test. The distribution of total and respirable fraction values was close to normal, and other data were also normally distributed. Therefore, parametric (one-way ANOVA, followed by post-hoc Tukey’s test) tests were applied to assess the significance of differences in the concentrations of bioaerosols between enclosures for different animals. Spearman’s correlation coefficients were applied to assess whether there are statistically significant relationships between the concentrations of fungal aerosol and physicochemical parameters of air in the tested sites. Cluster analysis (CA) and principal component analysis (PCA) were performed for the premises for each group of animals and for all premises grouped together using all variables tested in the experiment. Euclidean distance was adopted as a measure of similarity. Ward’s agglomerative clustering was adopted as a grouping method. In the PCA, two most important factors for each premise were selected for the results interpretation.
3 Results
Table 2 presents ranges and median concentrations of molds divided into different bioaerosol fractions. The lowest median of the total concentration of molds was recorded in rooms for pygmy hippopotamus 966 CFU/m3 (range 840–1325 CFU/m3). It is worth noting that this concentration was lower than the one recorded at the control site situated in the open area of the Zoological Garden (1.63 × 103 CFU/m3, range 1.54 × 103–1.82 × 103 CFU/m3). Similar concentrations were found in the rooms for tapirs (median = 1.5 × 103 CFU/m3, range 9.62 × 102–1.72 × 103 CFU/m3) and pheasants (median = 1.7 × 103 CFU/m3, range 1.54 × 103–1.83 × 103 CFU/m3). The highest median concentration of fungi was recorded in the exotarium, and it reached 1.68 × 104 CFU/m3 (range 9.79 × 103–2.84 × 104 CFU/m3), which was over 10 times higher compared to the control site. Statistically significant differences (p < 0.05) in the concentrations of total fungal aerosols were observed between most of the examined premises. Detailed results of post-hoc statistical analysis are shown in Table 3. The concentrations of fungal aerosol recorded in premises for ostriches and exotarium appeared to be significantly different from nearly all other sampling sites.
In the case of birds analyzed in our study, the median values differed considerably between pheasants and ostriches, as in the former group the median value for the total fungal aerosol was approx. 1.7 × 103 CFU/m3 (range of 1.5–1.8 × 103 CFU/m3), while for the latter the median was approx. 9.8 × 103 CFU/m3 (range of 7.3–17.1 × 103 CFU/m3).
The share of respirable fraction of fungal aerosol in the examined animal houses was highly variable. It ranged from 62.6% for the monkey rooms, to 89.2% for the exotarium, while 68.4% was recorded for the control site (atmospheric air) (Table 2).
As shown in Fig. 2, the particle size distribution of fungal aerosols in the premises for animals is very diverse; however, the fraction of 3.3–2.1 μm is predominant. The largest and the finest fractions have the smallest share. The distribution of particle size in rooms for hippos is the most uneven, i.e., nearly 60% consists of two fractions only (3.3–2.1 μm and 2.1–1.1 μm), whereas two other fractions consist of less than 12% (11.7 μm and 7–4.7 μm, Fig. 2). The opposite observations were made for fungal aerosol in the premises for monkeys—the share of individual fractions is the most even and ranges from 11.8 to 19.5%.
Considering the nature of the examined premises, the particulate matter concentration in the air was relatively low (Table 4; Figs. 3, 4). In the rooms for meerkats, the particulate matter concentration was lower (median for PM10–PM1 in the range from 0.052 to 0.062 mg/m3) than in the control site (medians of 0.074–0.089 mg/m3); on the other hand, in rooms for ostriches and pheasants the PM concentration was not much higher than in the control site. The highest concentration was recorded for PM10, PM4 and PM2.5 in the rooms for tapirs (medians of 0.165–0.186 mg/m3), in the exotarium (0.159–0.17 mg/m3) and in the rooms for hippos (0.152–0.163 mg/m3). The highest concentration of the finest particulate matter fraction (PM1) was observed in exotarium (0.141 mg/m3).
Despite the fact that the analyses were conducted in the cold season, the lowest recorded temperature (Fig. 5) was 17.2 °C, in the rooms for ostriches, with the median value of 22.2 °C. The highest temperatures and the smallest fluctuations were noted in the exotarium (26.2–27.6 °C). Similarly, the highest median and the smallest fluctuations of humidity were observed in exotarium (median of 85.3%, range of 84.7–86%; Fig. 6). Generally, the relative humidity in the animal premises varied from 58.7% observed in rooms for giraffes to 86.4% in rooms for ostriches (Fig. 6). Correlation analysis (Table 5), as well as the cluster analysis (Fig. 7), indicated that the concentrations of all fractions of bioaerosol as well as the total fraction were positively associated with the relative humidity of air. In the case of principal component analysis (Table 6) conducted for all animal enclosures grouped together and for the enclosures of individual animals, such relationship was confirmed in the case of all sites analyzed together, the enclosures for giraffes, meerkats, monkeys and ostriches. For the premises of other animals, the analysis allowed to distinguish also other factors that could have affected the fungal bioaerosol levels. The higher concentrations of bioaerosols were associated with higher particulate matter levels in the rooms for tapirs, giraffes, pheasants and ostriches. Higher fungal bioaerosol concentrations were on the other hand associated with lower temperatures in the rooms for giraffes, monkeys and ostriches.
4 Discussion
The majority of the measured concentrations of airborne molds in animal premises were below the limit value proposed by the Team of Experts in Biological Factors (Polish: ZECB), which in the case of fungi is 5 × 104 CFU/m3 of air. The threshold values were recommended by the ZECB based on cross-sectional environmental research, taking into consideration the potential harmfulness of a given biological agent. In order to do this, the levels of concentration of biological factors, usually occurring in public utility premises, are determined based on repeated measurements. Those are used to determine what levels are “typical and acceptable” for a given environment and what is “unusual and unacceptable”. Then, each measured value, if exceeds this usually occurring one, is treated as unusual and indicates the possibility of an additional source of contamination. The recommendations do not apply to specific animals or people, but to two groups of premises—work rooms polluted with organic dust and to residential and public utility facilities (Górny et al. 2011; Górny 2019). Only in two cases, i.e., in the enclosures for ostriches and in exotarium, the values observed in autumn (1.71 × 104 CFU/m3 and 2.84 × 104 CFU/m3, respectively) exceeded the proposed limit value for fungal aerosol. Based on these observations, the air in the animal shelters was generally considered mycologically uncontaminated.
Unfortunately, there is lack of research results, which our results could be directly compared to; they can only be referred to the ones obtained for the livestock rooms. For example, Millner (2009) provides the results obtained using a six-stage Andersen impactor in pig farms, where the mean fungal concentration was 1.5 × 102 CFU/m3, while in our study the mean concentration of airborne molds in premises for all animals was 8.61 × 102 CFU/m3, which is several times higher. But, comparison of this value with the mean concentration obtained in rooms for tapirs (i.e., 2.37 × 102 CFU/m3), which are most similar to pigs, shows that this difference is much smaller. On the other hand, much higher values of fungal aerosol were observed by Matković et al. (2007) in the cowshed of dairy cows. Depending on the time of day, the mean concentration of fungi ranged from 5.23 × 104 CFU/m3 (at noon) to 8.35 × 104 CFU/m3 (in the morning). In the case of animal production facilities, definitely the highest concentrations of fungal aerosol are observed on poultry farms and they can even reach 1.2 × 106 CFU/m3 in winter and 2.6 × 106 CFU/m3 in summer (Ławniczek-Wałczyk et al. 2013).
It seems that the concentration of fungal aerosol can be affected not only by the type, but also by the age of buildings, as well as by the type of ventilation. Karwowska (2005) studied the concentration of fungi in old and new types of farm buildings. In the old-type buildings (over several dozen years old and with gravitational ventilation), the concentrations of airborne fungi were within the range of 2.2 × 103–8 × 103 CFU/m3, while in modern facilities (less than 10 years and with mechanical ventilation) the concentrations were higher and were in the range of 1 × 104–2 × 104 CFU/m3. Identical observations regarding fungal aerosol concentrations in different types of barns were made by Pawlak et al. (2008). In our study, similar relationships were found in the Kraków Zoological Garden, as among the studied animal premises the youngest buildings were occupied by giraffes (year of construction—2013) and the fungal aerosol concentrations observed in these premises were quite low, but not the lowest. In turn, the lowest concentrations of airborne molds were found in the premises occupied by pygmy hippopotamuses that were kept in rather old buildings (i.e., 20 years old at the time of the study).
Our observations indicate that the share of respirable fraction is not affected by the size of animals or their species. What is important is that the greater the share of respirable fraction in the total concentration of bioaerosol, the more small particles are present in the air which can reach the lower respiratory tract of animals, employees and the visitors of the zoological garden (Owen et al. 1992). As shown by Lacey and Dutkiewicz (1994), occupational exposure among people working in environments, where small-sized aerosols are formed, may result not only in infections related directly to the contact with microbial pathogens, but also may cause diseases associated with the exposure to mycotoxins and fungal glucans. The symptoms caused by the exposure to the respirable fraction of bioaerosols include bronchitis, obstructive pulmonary disease, allergic asthma, alveolitis or organic dust toxic syndrome (Owen et al. 1992). In most of the examined sites, the percentage share of respirable fungi in their total concentration was similar to those observed by other authors (e.g., 68.8% observed by Sowiak et al. (2012) in swine farm buildings or a very wide range of 12.5–100% observed by Dutkiewicz et al. (1994) in different types of animal buildings). The RF share was close to or exceeded 80% in three sites—85.6% in the enclosures for hippos, 78.4% in the enclosures for ostriches and 89.2% in exotarium. Brągoszewska et al. (2018) state that the share of respirable fraction exceeding 80% should be considered serious, as it may pose health threats to the people exposed.
The acceptable level of the PM10 fraction in atmospheric air is 0.05 mg/m3 (Regulation of the Minister of the Environment on the levels of certain substances in the air); therefore, in the control site it was exceeded by 50 to 100%. Normative values for indoor spaces have not been developed so far. According to the Regulation of the Polish Minister of Family, Labor and Social Policy (2018), the exposure limit for the breathable fraction of organic dust of animal and plant origin is 4 mg/m3 while for the respirable fraction it is 2 mg/m3. Neither of these two values were exceeded in our study. There are no data available in the literature on the concentration of dust in animal rooms in zoological gardens. However, after comparing the values observed in our study to those recorded in livestock rooms, it is clear that in the latter ones they are significantly higher. Particularly high particulate matter concentration can be observed on poultry farms in relation to the PM10 fraction—it can reach values from 1.4 to 15.2 mg/m3 (Viegas et al. 2013), which is from several to several dozen times higher than the one observed in the Kraków Zoological Garden. The concentrations of PM2.5 and PM1 fractions on poultry farms are similar to or lower than in the studied zoo (Viegas et al. 2013). Jacobson et al. (2003) found significant differences between rooms for different livestock species and at the same time significantly different values depending on the country. The highest dust concentrations were observed on poultry farms in the Netherlands, pig breeding was characterized by lower dust concentrations, and the lowest values were observed in dairy cowsheds in England. Nevertheless, the values observed in our study are rather similar to those reported from nonagricultural indoor environments (the particulate matter values measured in our study ranged from 0.048 to 0.204 mg/m3, while those reported by Schneider et al. (2003) range within 0.02–0.44 mg/m3). What also needs to be remembered is that in the winter the limit concentrations for PM10 in outdoor air in cities are exceeded even several times (Reizer and Juda-Rezler 2016; European Environment Agency 2017) and Kraków is among Polish cities with the worst atmospheric air quality.
In animal rooms, we can easily measure microclimate conditions which act as cooling factors, such as temperature, humidity and air movement, but we are not able to assess the “thermal well-being” of animals (Tombarkiewicz et al. 2008). The optimum range of temperatures recommended for the studied groups of animals is within 16–30 °C (Kołacz and Dobrzański 2006). A bigger problem than maintaining the comfortable temperature in animal shelters is ensuring the proper relative humidity of the air, because there are numerous sources of moisture. A part of it comes from the outside air, while the rest—directly from animals themselves, from wet floors, walls and food. Too high or too low humidity causes discomfort in animals. Excessive humidity provides optimal conditions for the proliferation of microorganisms and increases the degree of animal cooling. At low humidity, the skin and mucous membranes of animals may dry out, which increases the risk of infection due to their cracking. According to Kołacz and Dobrzański (2006), the optimal relative humidity is 50–80%. In most animal premises, the humidity was within this range. Only in the rooms for ostriches, the humidity was much higher, but the median value was optimal (78.9%). Exotarium is a specific place, due to the animals, which are kept therein (fish, amphibians, reptiles). Their requirements, both in terms of temperature and humidity, differ from those of birds and mammals; hence, the relative humidity observed therein (84.7–86%) was optimal for those groups of animals. All of the mentioned environmental variables may affect the concentration of bioaerosols. The results of correlation analysis (Table 5) conducted in our study indicated that the concentrations of all fractions and total fungal aerosol were positively correlated with the relative humidity of air. The results of the cluster analysis made for all enclosures grouped together, presented in the form of a dendrogram (Fig. 7), cluster the air humidity with the total fungal aerosol as well as all its fractions, suggesting a positive correlation between those parameters. Principal component analysis results (Table 6) show the positive association between fungal bioaerosol and relative humidity in the case of all premises grouped together, enclosures for giraffes, meerkats, monkeys and ostriches. These observations are similar to those made by Sowiak et al. (2012) in their study on the exposure to bioaerosols among swine farm workers. Also Kim et al. (2007) suggested that the increase in indoor relative humidity facilitates the growth and proliferation of molds, thus resulting in higher concentrations of these microorganisms. Another observation made in our study was that most of the fine fractions of fungal aerosol were positively correlated with the concentration of fine particulate matter—PM1, and it was also similar to the observations by Sowiak et al. (2012) or Kim et al. (2007), who concluded that this might indicate a similarity of conditions facilitating the release of fungi and dust particles into the air. The temperature seems not to be among the most important factors affecting the fungal bioaerosol concentrations. The PCA results (Table 6) indicated a negative relationship between indoor temperature and bioaerosol levels in the enclosures for giraffes and ostriches, varied in the case of monkeys and a positive relationship in the enclosures for pheasants. One of the possible explanations of such situation might be the fact that varying outdoor temperature does not affect indoor temperature, especially in the sites where the cooling and heating systems are used throughout the year, and the temperature is a rather constant parameter (Chao et al. 2002).
What also needs to be mentioned is that the culture-based method used in our study has its limitations. For instance, as observed by Harkawy et al. (2011), the culturable airborne microorganisms constituted only from 0.5 to 3.9% of the total microflora. Also Niesler et al. (2010) observed that only a small percentage of microorganisms can be cultured by standard techniques; therefore, culture-based monitoring may underestimate the actual bioaerosol exposure. This is why research aimed at assessing the microbiological quality of air in public spaces, such as the studied Zoological Garden, should be supplemented by, e.g., molecular techniques, which would allow to identify also non-culturable microorganisms.
5 Conclusions
The levels of particulate matter observed in our study were relatively low and did not exceed the limit values proposed by the Polish authorities with respect to the environmental exposure of humans. Also the concentrations of airborne molds, even though differed significantly between the examined sites, were relatively low with the highest median values observed for the enclosures for ostriches and in exotarium. On the other hand, the share of respirable fraction was high in the case of houses for all groups of animals—also the highest in exotarium (nearly 90%), which indicates that even though we might conclude that the observed bioaerosol concentration does not pose particular health threats to the exposed workers or tourists of the Kraków zoological garden, special attention should be paid to thorough cleaning of animals and their premises, as well as to maintaining appropriate levels of microclimatic parameters in the rooms. Relative humidity appeared to be the factor that most significantly affected the concentration of total fungal aerosol and all its fractions. This was followed by the particulate matter levels. Temperature seems to be the factor that is least significant in shaping the bioaerosol concentrations.
Regardless of the undoubted shortcomings of the culture-based method used in our study, research of this type has not been conducted in environments as zoological gardens. Therefore, even preliminary results, such as those obtained in our study, may provide basic information for policy makers of bodies issuing indoor air quality standards about the typical concentrations of fungal aerosols in animal premises of zoological gardens. Also, the operators of zoological gardens could benefit from information obtained in such study, so as to be able to modernize ventilation devices or to implement tools to ensure proper microclimatic conditions that would facilitate efficient management of the potential environmental and public health risks.
References
Augustyńska, D., & Pośniak, M. (Ed.) (2016). Harmful factors in the working environment—Limit values. Interdepartmental Commission for Maximum Admissible Concentrations and Intensities for Agents Harmful to Health in the Working Environment: CIOP-PIB (in Polish).
Brągoszewska, E., Mainka, A., Pastuszka, J. S., Lizończyk, K., & Desta, Y. G. (2018). Assessment of bacterial aerosol in a preschool, primary school and high school in Poland. Atmosphere, 9(87), https://doi.org/10.3390/atmos9030087.
Budzińska, K., Szejniuk, B., Jurek, A., Traczykowski, A., Michalska, M., & Berleć, K. (2014). Microbial air contamination in the piggery. Problems of Agricultural Engineering, X-XII,4(86), 91–100. (in Polish).
Butler, D. A., & Madhavan, A. (2017). Communicating the health effects of indoor exposure to particulate matter. Indoor Air,27(3), 503–505.
Chao, H. J., Schwartz, J., Milton, D. K., & Burge, H. A. (2002). Populations and determinants of airborne fungi in large office buildings. Environmental Health Perspectives,110(8), 777–782.
Douglas, P., Robertson, S., Gay, R., Hansell, A. L., & Gant, T. W. (2018). A systematic review of the public health risks of bioaerosols from intensive farming. International Journal of Hygiene and Environmental Health,221(2), 134–173.
Dutkiewicz, J., Pomorski, Z. J. H., Sitkowska, J., Krysińska-Traczyk, E., Skórska, C., Prażmo, Z., et al. (1994). Airborne microorganisms and endotoxin in animal houses. Grana,33(2), 85–90.
European Environment Agency. (2017). Air quality in Europe-2017 report, Publications Office of the European Union, ISSN: 1977-8449.
Górny, R. L. (2019). Microbial aerosols: Sources, properties, health effects, exposure assessment—A review. KONA Powder and Particle Journal. https://doi.org/10.14356/kona.2020005.
Górny, R. L., Cyprowski, M., Ławniczek-Wałczyk, A., Gołofit-Szymczak, M., & Zapór, L. (2011). Biohazards in the indoor environment—A role for threshold limit values in exposure assessment. In M. R. Dudzińska (Ed.), The management of indoor air quality (pp. 1–20). London: CRC Press.
Grzyb, J., & Lenart-Boroń, A. (2019). Bacterial bioaerosol concentration and size distribution in the selected animal premises in a zoological garden. Aerobiologia,35(2), 253–268.
Harkawy, A., Górny, R. L., Ogierman, L., Wlazło, A., Ławniczek-Wałczyk, A., & Niesler, A. (2011). Bioaerosol assessment in naturally ventilated historical library building with restricted personnel access. Annals of Agricultural and Environmental Medicine,18(2), 323–329.
Jacobson, L. D., Bicudo, J. B., Schmidt, D. R., Wood-Gay, S., Gates, R. S., & Hoff, S. J. (2003). Air emissions from animal production buildings. In Proceedings of XIth International Congress in Animal Hygiene, 23–27 February 2003, Mexico City Autonomous Metropolitan University.
Karwowska, E. (2005). Microbiological air contamination in farming environment. Polish Journal of Environmental Studies,14(4), 445–449.
Kim, K. Y., Ko, H. J., Kim, H. T., Kim, Y. S., Roh, Y. M., Lee, C. M., et al. (2007). Influence of extreme seasons on airborne pollutant levels in a pig-confinement building. Archives of Environmental and Occupational Health,62(1), 27–32.
Kołacz, R., & Dobrzański, Z. (2006). Hygiene and welfare of livestock animals. Wrocław, Poland: AR Wrocław Publishing House (in Polish).
Lacey, J., & Dutkiewicz, J. (1994). Bioaerosols and occupational lung disease. Journal of Aerosol Science,25(8), 1371–1404.
Ławniczek-Wałczyk, A., Górny, R. L., Gołofit-Szymczak, M., Niesler, A., & Wlazlo, A. (2013). Occupational exposure to airborne microorganisms, endotoxins and β-glucans in poultry houses at different stages of the production cycle. Annals of Agricultural and Environmental Medicine,20(2), 259–268.
Matković, K., Vučemilo, M., Vinković, B., Pavičić, Ž., Matković, S., & Benić, M. (2009). Airborne fungi in a dairy barn with emphasis on microclimate and emissions. Veterinarski Arhiv,79, 207–218.
Matković, K., Vučemilo, M., Vinković, B., Šeol, B., Pavičić, Ž., & Matković, S. (2007). Qualitative structure of airborne bacteria and fungi in dairy barn and nearby environment. Czech Journal of Animal Science,52(8), 249–254.
Millner, P. D. (2009). Bioaerosols associated with animal production operations. Bioresource Technology,100, 5379–5385. https://doi.org/10.1016/j.biortech.2009.03.026.
Niesler, A., Górny, R. L., Wlazło, A., Łudzeń-Izbińska, B., Ławniczek-Wałczyk, A., Gołofit-Szymczak, M., et al. (2010). Microbial contamination of storerooms at the Auschwitz-Birkenau Museum. Aerobiologia,26, 25–133.
Owen, M. K., Ensor, D. S., & Sparks, L. E. (1992). Airborne particle sizes and sources found in indoor air. Atmospheric Environment. Part A. General Topics.,26(12), 2149–2162.
Pawlak, K., Grzyb, J., Rakowska, A., Tombarkiewicz, B., & Niedziółka, J. (2008). The impact of the maintenance of cows on the sanitary conditions in dairy cattle barns. Ecology and Engineering,XVI(5A), 138–141. (in Polish).
Priyamvada, H., Singh, R. K., Akila, M., Ravikrishna, R., Verma, R. S., & Gunthe, S. S. (2017). Seasonal variation of the dominant allergenic fungal aerosols—One year study from southern Indian region. Scientific Reports. https://doi.org/10.1038/s41598-017-11727-7.
Regulation of the Minister of the Environment of 24 August 2012 on the levels of certain substances in the air. Journal of Laws of the Republic of Poland 2012 item 1031.
Regulation of the Ministry of Family, Labour and Social Policy of 12 June 2018 on the highest permissible concentrations and intensities of factors harmful to health in the work environment. Journal of Laws of the Republic of Poland 2018 item 1286.
Reizer, M., & Juda-Rezler, K. (2016). Explaining the high PM10 concentrations observed in Polish urban areas. Air Quality, Atmosphere & Health,9, 517–531.
Samadi, S., Wouters, I. M., & Heederik, D. J. J. (2013). A review of bio-aerosol exposures and associated health effects in veterinary practice. Annals of Agricultural and Environmental Medicine,20(2), 206–221.
Schneider, T., Sundell, J., Bischof, W., Bohgard, M., Cherrie, J. W., Clausen, P. A., et al. (2003). EuroPart. Airborne particles in the indoor environment. A European interdisciplinary review of scientific evidence on associations between exposure to particles in buildings and health effects. Indoor Air, 13, 38–48.
Sowiak, M., Bródka, K., Buczyńska, A., Cyprowski, M., Kozajda, A., Sobala, W., et al. (2012). An assessment of potential exposure to bioaerosol among swine farm workers with particular reference to airborne microorganisms in the respirable fraction under various breeding conditions. Aerobiologia,28(2), 121–133.
The Council of the European Union. (1999). Council Directive 1999/22/EC of 29 March 1999 relating to the keeping of wild animals in zoos. Brussels.
Tombarkiewicz, B., Sulińska, M., Grzyb, J., Pawlak, K., & Niedziółka, J. (2008). Evaluation of sanitary and hygienic conditions in the monkey house of the Krakow Zoological Garden. Ecology and Technology,16(5A), 182–185.
Viegas, S., Faisca, V. M., Dias, H., Clerigo, A., Carolino, E., & Viegas, C. (2013). Occupational exposure to poultry dust and effects on the respiratory system in workers. Journal of Toxicology and Environmental Health, Part A,76, 230–239. https://doi.org/10.1080/15287394.2013.757199.
Webster, J. (1994). Assessment of animal welfare: The five freedoms. In J. Webster (Ed.), Animal welfare: A cool eye towards Eden (pp. 10–14). Oxford: Blackwell Science.
Acknowledgements
This study was funded by the statutory measures of the University of Agriculture—Grant No. DS 3158/KM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grzyb, J., Lenart-Boroń, A. Size distribution and concentration of fungal aerosol in animal premises of a zoological garden. Aerobiologia 36, 233–248 (2020). https://doi.org/10.1007/s10453-020-09625-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-020-09625-z