Abstract
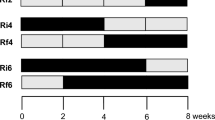
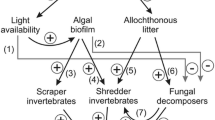
Intermittent streams, dominant in arid and semi-arid regions, are considered to be more representative of global river networks than perennial rivers. The impacts of constant changes in hydrological regime on the functioning of these streams and associated riparian areas does, however, remain to be elucidated. In this study, litter derived from two deciduous tree species (chestnut and oak) was used to compare microbial–decomposition patterns between an intermittent stream channel and its riparian area over a 1-year period. The stream channel exhibited higher decomposition rates than the riparian area for litter from both species, and higher fungal biomass only for chestnut. Despite a prolonged absence of streambed surface water (254 days), differences in hydrological conditions in the wetter seasons (autumn and winter) shape the decomposition dynamics in both zones throughout the whole hydrological cycle. The results point out the importance of the “hydrological imprint” for the leaves’ degradation; long-term studies are advisable over short-term ones to better understand the functioning of intermittent streams.



Similar content being viewed by others
Data availability
The authors are pleasured to share the data after formal request.
References
Abril M, Muñoz I, Menéndez M (2016) Heterogeneity in leaf litter decomposition in a temporary Mediterranean stream during flow fragmentation. Sci Total Environ 553:330–339. https://doi.org/10.1016/j.scitotenv.2016.02.082
Acuña V, Muñoz I, Giorgi A et al (2005) Drought and postdrought recovery cycles in an intermittent Mediterranean stream: structural and functional aspects. J North Am Benthol Soc 24:919–933. https://doi.org/10.1899/04-078.1
Arce MI, Mendoza-Lera C, Almagro M et al (2019) A conceptual framework for understanding the biogeochemistry of dry riverbeds through the lens of soil science. Earth-Sci Rev 188:441–453
Arias-Real R, Menéndez M, Abril M et al (2018) Quality and quantity of leaf litter: both are important for feeding preferences and growth of an aquatic shredder. PLoS ONE 13:e0208272. https://doi.org/10.1371/journal.pone.0208272
Arroita M, Flores L, Larrañaga A et al (2018) Hydrological contingency: drying history affects aquatic microbial decomposition. Aquat Sci 80:31. https://doi.org/10.1007/s00027-018-0582-3
Baldy V, Chauvet E, Charcosset J, Gessner MO (2002) Microbial dynamics associated with leaves decomposing in the mainstream and floodplain pond of a large river. Aquat Microb Ecol 28:25–36. https://doi.org/10.3354/ame028025
Bastias E, Ribot M, Romaní AM et al (2018) Responses of microbially driven leaf litter decomposition to stream nutrients depend on litter quality. Hydrobiologia 806:333–346. https://doi.org/10.1007/s10750-017-3372-3
Bhatnagar JM, Peay KG, Treseder KK (2018) Litter chemistry influences decomposition through activity of specific microbial functional guilds. Ecol Monogr 88:429
Boyero L, Pearson RG, Gessner MO et al (2011) A global experiment suggests climate warming will not accelerate litter decomposition in streams but might reduce carbon sequestration. Ecol Lett 14:289–294. https://doi.org/10.1111/j.1461-0248.2010.01578.x
Brandt LA, King JY, Hobbie SE et al (2010) The role of photodegradation in surface litter decomposition across a grassland ecosystem precipitation gradient. Ecosystems 13:765–781. https://doi.org/10.1007/s10021-010-9353-2
Bruder A, Chauvet E, Gessner MO (2011) Litter diversity, fungal decomposers and litter decomposition under simulated stream intermittency. Funct Ecol 25:1269–1277. https://doi.org/10.1111/j.1365-2435.2011.01903.x
Canhoto C, Calapez R, Gonçalves AL, Moreira-Santos M (2013) Effects of Eucalyptus leachates and oxygen on leaf-litter processing by fungi and stream invertebrates. Freshw Sci 32:411–424. https://doi.org/10.1899/12-062.1
Cebrian J (1999) Patterns in the fate of production in plant communities. Am Nat 154:449–468
Chauvet E, Cornut J, Sridhar KR et al (2016) Beyond the water column: aquatic hyphomycetes outside their preferred habitat. Fungal Ecol 19:112–127. https://doi.org/10.1016/j.funeco.2015.05.014
Cornut J, Ferreira V, Gonçalves AL et al (2015) Fungal alteration of the elemental composition of leaf litter affects shredder feeding activity. Freshw Biol 60:1755–1771
Corti R, Datry T, Drummond L, Larned ST (2011) Natural variation in immersion and emersion affects breakdown and invertebrate colonization of leaf litter in a temporary river. Aquat Sci 73:537–550
Datry T, Boulton AJ, Bonada N et al (2018) Flow intermittence and ecosystem services in rivers of the Anthropocene. J Appl Ecol 55:353–364. https://doi.org/10.1111/1365-2664.12941
Datry T, Corti R, Claret C, Philippe M (2011) Flow intermittence controls leaf litter breakdown in a French temporary alluvial river: the “drying memory.” Aquat Sci 73:471–483
Datry T, Larned ST, Tockner K (2014) Intermittent rivers: A challenge for freshwater ecology. Bioscience 64:229–235. https://doi.org/10.1093/biosci/bit027
Dieter D, von Schiller D, García-Roger EM et al (2011) Preconditioning effects of intermittent stream flow on leaf litter decomposition. Aquat Sci 73:599–609. https://doi.org/10.1007/s00027-011-0231-6
Dupont S, Lemetais G, Ferreira T et al (2012) Ergostol biosynthesis: a fungal pathway for life on land. Evolution 66:2961–2968. https://doi.org/10.5061/dryad.pd28pm7n
Ferreira V, Chauvet E (2011) Synergistic effects of water temperature and dissolved nutrients on litter decomposition and associated fungi. Glob Chang Biol 17:551–564. https://doi.org/10.1111/j.1365-2486.2010.02185.x
Ferreira V, Graça MS, de Lima JLMP, Gomes R (2006) Role of physical fragmentation and invertebrate activity in the breakdown rate of leaves. Arch für Hydrobiol 165:493–513. https://doi.org/10.1127/0003-9136/2006/0165-0493
Franken RJM, Waluto B, Peeters ETHM et al (2005) Growth of shredders on leaf litter biofilms: the effect of light intensity. Freshw Biol 50:459–466
Gessner MO, Chauvet E (1993) Ergosterol-to-biomass conversion factors for aquatic hyphomycetes. Appl Environ Microbiol 59:502–507
Gessner MO, Chauvet E, Dobson M (1999) A perspective on leaf litter breakdown in streams. Oikos 85:377–384
Gionchetta G, Oliva F, Romaní AM, Bañeras L (2020) Hydrological variations shape diversity and functional responses of streambed microbes. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.136838
Gonçalves AL, Graça MAS, Canhoto C (2015) Is diversity a buffer against environmental temperature fluctuations? - A decomposition experiment with aquatic fungi. Fungal Ecol 17:96–102. https://doi.org/10.1016/j.funeco.2015.05.013
Gonçalves AL, Lírio AV, Graça MAS, Canhoto C (2016) Fungal species diversity affects leaf decomposition after drought. Int Rev Hydrobiol 101:78–86. https://doi.org/10.1002/iroh.201501817
Gonçalves AL, Simões S, Bärlocher F, Canhoto C (2019) Leaf litter microbial decomposition in salinized streams under intermittency. Sci Total Environ 653:1204–1212. https://doi.org/10.1016/j.scitotenv.2018.11.050
Graça MAS, Ferreira RCF (1995) The ability of selected aquatic hyphomycetes and terrestrial fungi to decompose leaves in freshwater. Sydowia 47:167–179
Harms TK, Grimm NB (2012) Responses of trace gases to hydrologic pulses in desert floodplains. J Geophys Res Biogeosciences. https://doi.org/10.1029/2011JG001775
Hutchens JJJ, Wallace JB (2002) Ecosystem linkages between southern Appalachian headwater streams and their banks: Leaf litter breakdown and invertebrate assemblages. Ecosystems 5:80–91. https://doi.org/10.1007/s10021-001-0057-5
Jabiol J, Lecerf A, Lamothe S et al (2019) Litter quality modulates effects of dissolved nitrogen on leaf decomposition by stream microbial communities. Microb Ecol. https://doi.org/10.1007/s00248-019-01353-3
Kohl L, Myers-Pigg A, Edwards KA et al (2020) Microbial inputs at the litter layer translate climate into altered organic matter properties. Glob Chang Biol. https://doi.org/10.1111/gcb.15420
Kuehn KA (2016) Lentic and lotic habitats as templets for fungal communities: Traits, adaptations, and their significance to litter decomposition within freshwater ecosystems. Fungal Ecol 19:135–154. https://doi.org/10.1016/j.funeco.2015.09.009
Kuehn KA, Francoeur SN, Findlay RH, Neely RK (2014) Priming in the microbial landscape: periphytic algal stimulation of litter-associated microbial decomposers. Ecology 95:749–762
Langhans SD, Tockner K (2006) The role of timing, duration, and frequency of inundation in controlling leaf litter decomposition in a river-floodplain ecosystem (Tagliamento, northeastern Italy). Oecologia 147:501–509. https://doi.org/10.1007/s00442-005-0282-2
Lecerf A, Chauvet E (2008) Intraspecific variability in leaf traits strongly affects alder leaf decomposition in a stream. Basic Appl Ecol 9:598–605. https://doi.org/10.1016/j.baae.2007.11.003
LeRoy CJ, Fischer DG, Halstead K et al (2011) A fungal endophyte slows litter decomposition in streams. Freshw Biol 56:1426–1433. https://doi.org/10.1111/j.1365-2427.2011.02581.x
Lohse KA, Gallo EL, Meixner T (2020) Influence of climate and duration of stream water presence on rates of litter decomposition and nutrient dynamics in temporary streams and surrounding environments of southwestern USA. Front Water 2:571044
Maamri A, Bärlocher F, Pattee E, Chergui H (2001) Fungal and bacterial colonisation of Salix pedicellata leaves decaying in permanent and intermittent streams in Eastern Morocco. Int Rev Hydrobiol 86:337–348
Martínez A, Pérez J, Molinero J et al (2015) Effects of flow scarcity on leaf-litter processing under oceanic climate conditions in calcareous streams. Sci Total Environ 503:251–257
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76:151–174. https://doi.org/10.1890/0012-9615(2006)076[0151:ATMOLD]2.0.CO;2
Mora-Gómez J, Boix D, Duarte S et al (2020) Legacy of summer drought on autumnal leaf litter processing in a temporary mediterranean stream. Ecosystems 23:989–1003. https://doi.org/10.1007/s10021-019-00451-0
Mora-Gómez J, Duarte S, Cássio F et al (2018) Microbial decomposition is highly sensitive to leaf litter emersion in a permanent temperate stream. Sci Total Environ 621:486–496. https://doi.org/10.1016/j.scitotenv.2017.11.055
Mori N, Simčič T, Brancelj A et al (2017) Spatiotemporal heterogeneity of actual and potential respiration in two contrasting floodplains. Hydrol Process 31:2622–2636
Niyogi DK, Hu C-Y, Vessell BP (2020) Response of stream fungi on decomposing leaves to experimental drying. Int Rev Hydrobiol 105:52–58
Petersen RC, Cummins KW (1974) Leaf processing in a woodland stream. Freshw Biol 4:343–368
Pieristè M, Chauvat M, Kotilainen TK et al (2019) Solar UV-A radiation and blue light enhance tree leaf litter decomposition in a temperate forest. Oecologia 191:191–203. https://doi.org/10.1007/s00442-019-04478-x
Pinna M, Basset A (2004) Summer drought disturbance on plant detritus decomposition processes in three River Tirso (Sardinia, Italy) sub-basins. Hydrobiologia 522:311–319
Pozo J, Casas J, Menéndez M et al (2011) Leaf-litter decomposition in headwater streams: a comparison of the process among four climatic regions. J North Am Benthol Soc 30:935–950. https://doi.org/10.1899/10-153.1
R Development Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raymond PA, Hartmann J, Lauerwald R et al (2013) Global carbon dioxide emissions from inland waters. Nature 503:355–359. https://doi.org/10.1038/nature12760
Razavi BS, Liu S, Kuzyakov Y (2017) Hot experience for cold-adapted microorganisms: temperature sensitivity of soil enzymes. Soil Biol Biochem 105:236–243. https://doi.org/10.1016/j.soilbio.2016.11.026
Romaní AM, Chauvet E, Febria C et al (2017) The biota of intermittent rivers and ephemeral streams: prokaryotes, fungi, and protozoans. Elsevier Inc, Amsterdam
Schneider A, Jost A, Coulon C et al (2017) Global-scale river network extraction based on high-resolution topography and constrained by lithology, climate, slope, and observed drainage density. Geophys Res Lett 44:2773–2781
Skoulikidis NT, Sabater S, Datry T et al (2017) Non-perennial Mediterranean rivers in Europe: status, pressures, and challenges for research and management. Sci Total Environ 577:1–18. https://doi.org/10.1016/j.scitotenv.2016.10.147
Sridhar KR, Bärlocher F (1993) Aquatic hyphomycetes on leaf litter in and near a stream in Nova Scotia, Canada. Mycol Res 97:1530–1535. https://doi.org/10.1016/S0953-7562(09)80229-3
Steward AL, Von Schiller D, Tockner K et al (2012) When the river runs dry: human and ecological values of dry riverbeds. Front Ecol Environ 10:202–209. https://doi.org/10.1890/110136
von Schiller D, Bernal S, Dahm CN, Martí E (2017) Nutrient and organic matter dynamics in intermittent rivers and ephemeral streams. Intermittent rivers and ephemeral streams: ecology and management. Elsevier Inc., Amsterdam, pp 135–160
Wallace JB, Eggert SL, Meyer JL, Webster JR (1997) Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 277:102–104
Acknowledgements
This study was supported by FCT, within the POCH – Human Capital Operating Program, to SS (Fellowship Reference SFRH/BD/119133/2016), co-funded by the European Social Fund and MCTES national funds, is gratefully acknowledged. Also financed by a) Project UID/BIA/04004/2013 co-funded by FCT/MEC through national funds and by FEDER, within the PT2020 Partnership Agreement, and COMPETE 2020; and b) Project ReNATURE—Valorization of the Natural Endogenous Resources of the Centro Region (Centro 2020, Centro-01-0145-FEDER-000007), which also supported AM (fellowship reference ReNATURE – BPD11_2). NC was supported by the PhD Grant SFRH/BD/133352/2017 and JA by the post doc Grant SFRH/BPD/123087/2016.
Author information
Authors and Affiliations
Contributions
Design of the experiment was done by ALG, THJ, JPS, CC. Field procedures were carried out by SS, NC, JA, AAS. Laboratory procedures were carried out by SS, ALG. Data treatment was done by SS, AM. Writing the original draft was done by SS, AM, CC. Review of the draft was done by SS, AM, ALG, NC, JA, AAS, THJ, JPS, CC.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
The authors consent to participate in peer review process.
Consent for publication
The authors consent for publication in the terms of the journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Télesphore Sime-Ngando.
Rights and permissions
About this article
Cite this article
Simões, S., Martínez, A., Gonçalves, A.L. et al. Annual patterns of litter decomposition in the channel and riparian areas of an intermittent stream. Aquat Ecol 55, 519–526 (2021). https://doi.org/10.1007/s10452-021-09841-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-021-09841-w