Abstract
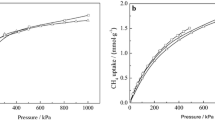
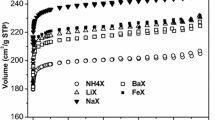
In natural gas sweetening processes, expensive technologies are usually applied, where adsorption can be a viable and economic alternative. In this sense, the usage of adsorption for natural gas sweetening depends on adsorbent potentiality. Thus, in this work, the potential usage of FAU NaX and NaY for natural gas sweetening at high-pressure (4 MPa) and ambient temperature (298 K) by adsorption processes was investigated by simulations using a validated Multicomponent Potential Theory of Adsorption coupled to Dubinin–Radushkevitch–Astakhov model (MPTA-DRA). Pure component adsorption data for H2S, CO2, CH4 and N2 show favorable isotherms on both materials and the correlation using MPTA-DRA model is in good agreement with experimental results. The simulation of multicomponent sour natural gas adsorption indicates that almost none CH4 and N2 are adsorbed in conditions studied and that CO2 and H2S compete for adsorption sites on both NaX and NaY. Partition coefficients of H2S are higher than CO2 and increase from 18.90 to 43.30, for NaX, and from 4.04 to 27.39, for NaY, as CO2 molar fraction in bulk phase decreases. The selectivity of H2S over CO2 follows a different trend, decreasing from 27.16 to 20.14 for NaX and remaining somewhat constant around 4 for NaY. These results suggest higher selectivity for NaX when a lower CO2 molar gas fraction is present. Meanwhile, for NaY, the CO2 molar fraction does not influence the H2S/CO2 selectivity. Therefore, simulations indicate that FAU NaX and NaY have good potential for natural gas sweetening at 4 MPa and 298 K.




Similar content being viewed by others
References
BPSTATS: BP Statistical Review of World Energy Statistical Review of World, 68th edition. Ed. BP Stat. Rev. World Energy. pp. 1–69 (2019)
Alcheikhhamdon, Y., Hoorfar, M.: Natural gas quality enhancement: a review of the conventional treatment processes, and the industrial challenges facing emerging technologies. J. Nat. Gas Sci. Eng. 34, 689–701 (2016). https://doi.org/10.1016/j.jngse.2016.07.034
Yang, R.T.: Adsorbents: Fundamentals and Applications. Wiley, Hoboken (2003)
de Oliveira, L.H., Meneguin, J.G., Pereira, M.V., do Nascimento, J.H., Arroyo, P.A.: Adsorption of hydrogen sulfide, carbon dioxide, methane, and their mixtures on activated carbon. Chem. Eng. Commun. 206, 1544–1564 (2019). https://doi.org/10.1080/00986445.2019.1601627
de Oliveira, L.H., Meneguin, J.G., Pereira, M.V., da Silva, E.A., Grava, W.M., do Nascimento, J.H., Arroyo, P.A.: H2S adsorption on NaY zeolite. Microporous Mesoporous Mater. 284, 247–257 (2019). https://doi.org/10.1016/j.micromeso.2019.04.014
Pinzan, F., Braga, M.U.C., de Carvalho, E.P., Pereira, M.V., de Oliveira, L.H., do Nascimento, J.H., Arroyo, P.A.: Parametric analysis of two-dimensional equation of state used to predict high pressure CO2 and CH4 on NaY zeolite and babassu activated carbon. Fluid Phase Equilib. 539, 113031 (2021). https://doi.org/10.1016/j.fluid.2021.113031
Wynnyk, K.G., Hojjati, B., Marriott, R.A.: High-pressure sour gas and water adsorption on zeolite 13X. Ind. Eng. Chem. Res. 57, 15357–15365 (2018). https://doi.org/10.1021/acs.iecr.8b03317
Siriwardane, R.V., Shen, M.S., Fisher, E.P., Losch, J.: Adsorption of CO2 on zeolites at moderate temperatures. Energy Fuels 19, 1153–1159 (2005). https://doi.org/10.1021/ef040059h
Karge, H.G., Raskó, J.: Hydrogen sulfide adsorption on faujasite-type zeolites with systematically varied Si-Al ratios. J. Colloid Interface Sci. 64, 522–532 (1978). https://doi.org/10.1016/0021-9797(78)90394-6
Keller, J., Staudt, R.: Gas Adsorption Equilibria - Experimental Methods and Adsorptive Isotherms. Springer, New York (2005)
Suzuki, M.: Adsorption Engineering. Elsevier Science B.V, Tokyo (1991)
Walton, K.S., Sholl, D.S.: Predicting multicomponent adsorption: 50 years of the ideal adsorbed solution theory. AIChE J. 61, 2757–2762 (2015). https://doi.org/10.1002/aic
Shapiro, A.A., Stenby, E.H.: Potential theory of multicomponent adsorption. J. Colloid Interface Sci. 201, 146–157 (1998). https://doi.org/10.1006/jcis.1998.5424
Bartholdy, S., Bjørner, M.G., Solbraa, E., Shapiro, A., Kontogeorgis, G.M.: Capabilities and limitations of predictive engineering theories for multicomponent adsorption. Ind. Eng. Chem. Res. 52, 11552–11563 (2013). https://doi.org/10.1021/ie400593b
Dong, X., Liu, H., Guo, W., Hou, J., Chen, Z., Wu, K.: Study of the confined behavior of hydrocarbons in organic nanopores by the potential theory. Fluid Phase Equilib. 429, 214–226 (2016). https://doi.org/10.1016/j.fluid.2016.09.008
Monsalvo, M.A., Shapiro, A.A.: Modeling adsorption of binary and ternary mixtures on microporous media. Fluid Phase Equilib. 254, 91–100 (2007). https://doi.org/10.1016/j.fluid.2007.02.006
Monsalvo, M.A., Shapiro, A.A.: Study of high-pressure adsorption from supercritical fluids by the potential theory. Fluid Phase Equilib. 283, 56–64 (2009). https://doi.org/10.1016/j.fluid.2009.05.015
Ren, W., Tian, S., Li, G., Sheng, M., Yang, R.: Modeling of mixed-gas adsorption on shale using hPC-SAFT-MPTA. Fuel 210, 535–544 (2017). https://doi.org/10.1016/j.fuel.2017.09.012
Tamburello, D., Hardy, B., Sulic, M.: Multi-component separation and purification of natural gas. In: ASME 2018 Power Conference, pp. 1–8 (2018)
Bjørner, M.G., Shapiro, A.A., Kontogeorgis, G.M.: Potential theory of adsorption for associating mixtures: possibilities and limitations. Ind. Eng. Chem. Res. 52, 2672–2684 (2013). https://doi.org/10.1021/ie302144t
Wang, Y., Helvensteijn, B., Nizamidin, N., Erion, A.M., Steiner, L.A., Mulloth, L.M., Luna, B., Levan, M.D.: High pressure excess isotherms for adsorption of oxygen and nitrogen in zeolites. Langmuir 27, 10648–10656 (2011). https://doi.org/10.1021/la201690x
Feng, L., Shen, Y., Wu, T., Liu, B., Zhang, D., Tang, Z.: Adsorption equilibrium isotherms and thermodynamic analysis of CH4, CO2, CO, N2 and H2 on NaY Zeolite. Adsorption 26, 1101–1111 (2020). https://doi.org/10.1007/s10450-020-00205-8
Nesterov, I., Shapiro, A., Kontogeorgis, G.M.: Multicomponent adsorption model for polar and associating mixtures. Ind. Eng. Chem. Res. 54, 3039–3050 (2015). https://doi.org/10.1021/acs.iecr.5b00208
Shapiro, A.A., Stenby, E.H.: High pressure multicomponent adsorption in porous media. Fluid Phase Equilib. 158–160, 565–573 (1999). https://doi.org/10.1016/S0378-3812(99)00144-2
Peng, D.Y., Robinson, D.B.: A new two-constant equation of state. Ind. Eng. Chem. Fundam. 15, 59–64 (1976). https://doi.org/10.1021/i160057a011
Burgers, W.F.J., Northrop, P.S., Kheshgi, H.S., Valencia, J.A.: Worldwide development potential for sour gas. Energy Procedia 4, 2178–2184 (2011). https://doi.org/10.1016/j.egypro.2011.02.104
Zheng, D., Pang, X., Luo, B., Chen, D., Pang, B., Li, H., Yu, R., Guo, F., Li, W.: Geochemical characteristics, genetic types, and source of natural gas in the Sinian Dengying Formation, Sichuan Basin, China. J. Pet. Sci. Eng. 199, 108341 (2021). https://doi.org/10.1016/j.petrol.2020.108341
Thommes, M., Kaneko, K., Neimark, A.V., Olivier, J.P., Rodriguez-Reinoso, F., Rouquerol, J., Sing, K.S.W.: Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87, 1051–1069 (2015). https://doi.org/10.1515/pac-2014-1117
Wynnyk, K.G., Hojjati, B., Pirzadeh, P., Marriott, R.A.: High-pressure sour gas adsorption on zeolite 4A. Adsorption 23, 149–162 (2017). https://doi.org/10.1007/s10450-016-9841-6
Castrillon, M.C., Moura, K.O., Alves, C.A., Bastos-Neto, M., Azevedo, D.C.S., Hofmann, J., Möllmer, J., Einicke, W.D., Gläser, R.: CO2 and H2S removal from CH4-Rich streams by adsorption on activated carbons modified with K2CO3, NaOH, or Fe2O3. Energy Fuels 30, 9596–9604 (2016). https://doi.org/10.1021/acs.energyfuels.6b01667
Olney, T.N., Cann, N.M., Cooper, G., Brion, C.E.: Absolute scale determination for photoabsorption spectra and the calculation of molecular properties using dipole sum-rules. Chem. Phys. 223, 59–98 (1997). https://doi.org/10.1016/S0301-0104(97)00145-6
Kiselev, A.V., Lopatkin, A.A., Shulga, A.A.: Molecular statistical calculation of gas adsorption by silicalite. Zeolites 5, 261–267 (1985). https://doi.org/10.1016/0144-2449(85)90098-3
Meek, S.T., Teich-McGoldrick, S.L., John, J.P.I., Greathouse, J.A., Allendorf, M.D.: Effects of polarizability on the adsorption of noble gases at low pressures in monohalogenated isoreticular metal−organic frameworks. J. Phys. Chem. C 116, 19765–19772 (2012). https://doi.org/10.1021/jp303274m
Yang, J., Li, J., Wang, W., Li, L., Li, J.: Adsorption of CO2, CH4, and N2 on 8-,10-, and 12-membered ring hydrophobic microporous high-silica zeolites: DDR, silicalite-1, and beta. Ind. Eng. Chem. Res. 52, 17856–17864 (2013)
Cui, X., Bustin, R.M., Dipple, G.: Selective transport of CO2, CH4, and N2 in coals: insights from modeling of experimental gas adsorption data. Fuel 83, 293–303 (2004). https://doi.org/10.1016/j.fuel.2003.09.001
Sigot, L., Ducom, G., Germain, P.: Adsorption of hydrogen sulfide (H2S) on zeolite (Z): retention mechanism. Chem. Eng. J. 287, 47–53 (2016). https://doi.org/10.1016/j.cej.2015.11.010
Du, X., Pang, D., Zhao, Y., Hou, Z., Wang, H., Cheng, Y.: Investigation into the adsorption of CO2, N2 and CH4 on kaolinite clay. Arab. J. Chem. 15, 103665 (2022). https://doi.org/10.1016/j.arabjc.2021.103665
Poling, B.E., Prausnitz, J.M., O’Connell, J.P.: The Properties of Gases and Liquids. McGraw-Hill, New York (2001)
Graham, C., Pierrus, J., Raab, R.E.: Measurement of the electric quadrupole moments of CO2, CO and N2. Mol. Phys. 67, 939–955 (1989). https://doi.org/10.1080/00268978900101551
Sirkecioğlu, A., Altav, Y., Erdem-Şenatalar, A.: Adsorption of H2S and SO2 on Bigadiç clinoptilolite. Sep. Sci. Technol. 30, 2747–2742 (1995)
Shao, W., Zhang, L., Li, L., Lee, R.L.: Adsorption of CO2 and N2 on synthesized NaY zeolite at high temperatures. Adsorption 15, 497–505 (2009). https://doi.org/10.1007/s10450-009-9200-y
Pulin, A.L., Fomkin, A.A.: Thermodynamics of CO2 adsorption on zeolite NaX in wide intervals of pressures and temperatures. Russ. Chem. Bull. 53, 1630–1634 (2004). https://doi.org/10.1007/s11172-005-0008-y
Cavenati, S., Grande, C.A., Rodrigues, A.E.: Adsorption equilibrium of methane, carbon dioxide, and nitrogen on zeolite 13X at high pressures. J. Chem. Eng. Data 49, 1095–1101 (2004). https://doi.org/10.1021/je0498917
Zhou, L., Bai, S., Su, W., Yang, J., Zhou, Y.: Comparative study of the excess versus absolute adsorption of CO2 on superactivated carbon for the near-critical region. Langmuir 19, 2683–2690 (2003). https://doi.org/10.1021/la020682z
Rudzinski, W., Everett, D.H.: Adsorption of Gases on Heterogeneous Surfaces. Academic Press, London (1992)
Hutson, N.D., Yang, R.T.: Theoretical basis for the Dubinin-Radushkevitch (D-R) adsorption isotherm equation. Adsorption 3, 189–195 (1997). https://doi.org/10.1007/BF01650130
Chakraborty, A., Sun, B.: An adsorption isotherm equation for multi-types adsorption with thermodynamic correctness. Appl. Therm. Eng. 72, 190–199 (2014). https://doi.org/10.1016/j.applthermaleng.2014.04.024
Acknowledgements
Authors would like to thank Petrobras for the financial support (Cooperation Number 5850.0102576.16.9).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pereira, M.V., de Oliveira, L.H., do Nascimento, J.F. et al. Simulation of high-pressure sour natural gas adsorption equilibrium on NaX and NaY zeolites using the multicomponent potential theory of adsorption. Adsorption 29, 65–72 (2023). https://doi.org/10.1007/s10450-022-00373-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-022-00373-9