Abstract
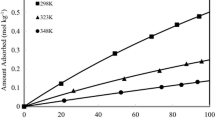
Adsorption capacities of CH4, CO2, CO, N2 and H2 on NaY zeolite were measured at 298 K, 318 K, 338 K and 358 K with pressure ranged from 0 to 10 bar. The order of adsorption capacity was CO2 ≫ CH4 > CO > N2 ≫ H2. The experiment data were fitted by the Langmuir, Toth and Sips equations. The fitting relativity of above models were also compared. Moreover, the isosteric heat of adsorption were calculated by the Clausius–Clapeyron equation, the results showed that the adsorption heat of CO2 is the largest (41.89764 kJ/mol with adsorption loading of 5.8 mmol/g) and that of H2 is the smallest. Finally, the selectivity of binary mixture was predicted according to the IAS theory.









Similar content being viewed by others
References
Bao, Z., Yu, L., Dou, T., Gong, Y., Zhang, Q., Ren, Q., Lu, X., Deng, S.: Adsorption equilibria of CO2, CH4, N2, O2 and Ar on high silica zeolites. J. Chem. Eng. Data 56(11), 4017–4023 (2011)
Choi, B., Choi, D., Lee, Y., Lee, B., Kim, S.: Adsorption equilibria of methane, ethane, ethylene, nitrogen, and hydrogen onto activated carbon. J. Chem. Eng. Data 48(3), 603–607 (2003)
Do, D.D.: Adsorption Analysis: Equilibria and Kinetics. Imperial College Press, London (1998)
Grande, C.A., Lopes, F.V.S., Ribeiro, A.M., Loureiro, J.M., Rodrigues, A.E.: Adsorption of off-gases from steam methane reforming (H2, CO2, CH4, CO and N2) on activated carbon. Sep. Sci. Technol. 43(6), 1338–1364 (2008)
Hua, D., Honghong, Y., Xiaolong, T., Ping, N., Qiongfen, Y., Liping, Y.: Equilibrium isotherms and thermodynamics for flue gases on 13X zeolite. J. Central South Univ. 43(01), 401–406 (2012)
Lopes, F.V.S., Grande, C.A., Ribeiro, A.M., Loureiro, J.M., Evaggelos, O., Nikolakis, V., Rodrigues, A.E.: Adsorption of H2, CO2, CH4, CO, N2 and H2O in activated carbon and zeolite for hydrogen production. Sep. Sci. Technol. 44(5), 1045–1073 (2009)
Nam, G., Jeong, B., Kang, S., Lee, B., Choi, D.: Equilibrium isotherms of CH4, C2H6, C2H4, N2, and H2 on zeolite 5A using a static volumetric method. J. Chem. Eng. Data 50(1), 72–76 (2005)
Pakseresht, S., Kazemeini, M., Akbarnejad, M.M.: Equilibrium isotherms for CO, CO2, CH4 and C2H4 on the 5A molecular sieve by a simple volumetric apparatus. Sep. Purif. Technol. 28(1), 53–60 (2002)
Park, Y., Moon, D., Kim, Y., Ahn, H., Lee, C.: Adsorption isotherms of CO2, CO, N2, CH4, Ar and H2 on activated carbon and zeolite LiX up to 1.0 MPa. Adsorption 20(4), 631–647 (2014)
Park, Y., Ju, Y., Park, D., Lee, C.: Adsorption equilibria and kinetics of six pure gases on pelletized zeolite 13X up to 1.0 MPa: CO2, CO, N2, CH4, Ar and H2. Chem. Eng. J. 292, 348–365 (2016)
Peng, X., Cao, D., Wang, W.: Adsorption and separation of CH4/CO2/N2/H2/CO mixtures in hexagonally ordered carbon nanopipes CMK-5. Chem. Eng. Sci. 66(10), 2266–2276 (2011)
Pengcheng, Y.: Adsorption Equilibrium of Ethylene and Propylene on Activated Carbons. Zhejiang University, Zhejiang (2011)
Son, K.N., Cmarik, G.E., Knox, A.C., Weibel, J.A., Garimella, U.V.: Measurement and prediction of the heat of adsorption and equilibrium concentration of CO2 on zeolite 13X. J. Chem. Eng. Data 43(41), 1663–1674 (2018)
Song, C., et al.: Hydrogen and syngas production and purification technologies. Pennsylvania State University, University Park (2009)
Su, W., Zhang, A., Sun, Y., Ran, M., Wang, X.: Adsorption properties of C2H4 and C3H6 on 11 adsorbents. J. Chem. Eng. Data 62(1), 417–421 (2016)
Wei, L., Linbing, S., Xiaoqin, L.: Adsorption of ethylene and carbon dioxide binary systems on active carbon: experiment and model. J. Chem. Eng. Chin. Univ. 26(01), 25–30 (2012)
Wenrong, S., Huawei, Y., Yuanhui, S., Qiang, F., Donghui, Z., Bo, F.: Two-stage PSA/VSA to produce H2 with CO2 capture via steam methane reforming (SMR). Int. J. Hydrog. Energy 43(41), 19057–19074 (2018)
Yang, J., Li, J., Wang, W., Li, L., Li, J.: Adsorption of CO2, CH4 and N2 on 8-, 10-, and 12-membered ring hydrophobic microporous high-silica zeolites: DDR, silicalite-1, and beta. Ind. Eng. Chem. Res. 52(50), 17856–17864 (2013)
Yu, H.: Adsorption and Separation of CO2 On Kureha Carbon and NaY. Zhejiang Normal University, Zhejiang (2015)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feng, L., Shen, Y., Wu, T. et al. Adsorption equilibrium isotherms and thermodynamic analysis of CH4, CO2, CO, N2 and H2 on NaY Zeolite. Adsorption 26, 1101–1111 (2020). https://doi.org/10.1007/s10450-020-00205-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-020-00205-8