Abstract
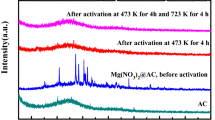
Zeolite Y supported MgO (denoted as MgO@Y) composites have been successfully prepared using Mg(NO3)2 as precursor via a facile solid-state heat dispersion approach. The samples are characterized by X-ray diffraction and N2 adsorption/desorption, and investigated for CO2 adsorption performance including adsorption capacity, adsorption selectivity and stability. The results reveal that MgO can be highly dispersed on the surfaces of zeolite Y support after the activation at high temperatures, and the monolayer dispersion capacity of MgO on zeolite Y support is 3 mmol/g zeolite Y. The resulting MgO(3.0)@Y adsorbent with the magnesium loadings of 3 mmol/g zeolite Y displays a high CO2 adsorption capacity of 2.78 mmol/g at 500 kPa, which is about 28% higher than that of zeolite Y support. Moreover, the MgO(3.0)@Y adsorbent displays a high CO2/N2 adsorption selectivity of 32 and a excellent cyclic stability. Its good performance as well as its facile preparation process make it attractive candidate for the adsorption of CO2 in flue gas vents. In addition, the isosteric heat of CO2 adsorption on the MgO(3.0)@Y sample was calculated from the Clausius–Clapeyron equation, and the values the isosteric heats of adsorption lie in the range of 27.8–20.0 kJ/mol.






Similar content being viewed by others
References
Akten, E.D., Siriwardane, R., Sholl, D.S.: Monte carlo simulation of single- and binary-component adsorption of CO2, N2, and H2 in Zeolite Na-4A. Energ. Fuel. 17, 977–983 (2003)
Avijegon, G., Xiao, G., Li, G., May, E.F.: Binary and ternary adsorption equilibria for CO2/CH4/N2 mixtures on Zeolite 13 × beads from 273 to 333 K and pressures to 900 kPa. Adsorption 24(4), 381–392 (2018)
Bahamon, D., Vega, L.F.: Systematic evaluation of materials for post-combustion CO2 capture in a temperature swing adsorption process. Chem. Eng. J. 284, 438–447 (2016)
Belmabkhout, Y., Sayari, A.: Adsorption of CO2 from dry gases on MCM-41 silica at ambient temperature and high pressure. 2: adsorption of CO2/N2, CO2/CH4 and CO2/H2 binary mixtures. Chem. Eng. Sci. 64, 3729–3735 (2009)
Billemont, P., Heymans, N., Normand, P., Weireld, G.D.: IAST predictions vs co-adsorption measurements for CO2 capture and separation on MIL-100 (Fe). Adsorption 23(2–3), 225–237 (2017)
Carruthers, J.D., Petruska, M.A., Sturm, E.A., Wilson, S.M.: Molecular sieve carbons for CO2 capture. Microporous Mesoporous Mater. 154, 62–67 (2012)
Chen, Y., Xie, C., Li, Y., Song, C., Bolin, T.B.: Sulfur poisoning mechanism of steam reforming catalysts: an X-ray absorption near edge structure (XANES) spectroscopic study. Phys. Chem. Chem. Phys. 12(21), 5707–5711 (2010)
Chen, C., Kim, J., Ahn, W.S.: CO2 capture by amine-functionalized nanoporous materials: a review. Korean J. Chem. Eng. 31(11), 1919–1934 (2014)
Choma, J., Stachurska, K., Marszewski, M., Jaroniec, M.: Equilibrium isotherms and isosteric heat for CO2 adsorption on nanoporous carbons from polymers. Adsorption 22(4), 581–588 (2016)
Chu, S.: Carbon capture and sequestration. Science 325(5948), 1599 (2009)
Dietzel, P.D.C., Besikiotis, V., Blom, R.: Application of metal-organic frameworks with coordinatively unsaturated metal sites in storage and separation of methane and carbon dioxide. J. Mater. Chem. 19, 7362–7370 (2009)
Dong, W., Chen, X., Yu, F., Wu, Y.: Na2CO3/MgO/Al2O3 solid sorbents for low-temperature CO2 capture. Energy Fuel. 29(2), 968–973 (2015)
Figueroa, J.D., Fout, T., Plasynski, S., McIlvried, H.: Advances in CO2 capture technology—The U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Con. 2(1), 9–20 (2008)
Freundlich, H.M.F.: Over the adsorption in solution. J. Phys. Chem. 57, 385–471 (1906)
Gao, F., Wang, Y., Wang, X., Wang, S.: Selective CO adsorbent CuCl/AC prepared using CuCl2 as a precursor by a facile method. RSC Adv. 6(41), 34439–34446 (2016a)
Gao, F., Wang, Y., Wang, S.: Selective adsorption of CO on CuCl/Y adsorbent prepared using CuCl2 as precursor: equilibrium and thermodynamics. Chem. Eng. J. 290, 418–427 (2016b)
Gao, F., Wang, Y., Wang, X., Wang, S.: Ethylene/ethane separation by Cu/AC adsorbent prepared using CuCl2 as a precursor. Adsorption 22(7), 1013–1022 (2016c)
Han, S.J., Bang, Y., Lee, H., Lee, K., Song, I.K., Seo, J.G.: Synthesis of a dual-templated MgO-Al2O3 adsorbent using block copolymer and ionic liquid for CO2 capture. Chem. Eng. J. 270, 411–417 (2015)
Hasana, M.M.F., First, E.L., Boukouvala, F., Flouda, C.A.: A multi-scale framework for CO2 capture, utilization, and sequestration: CCUS and CCU. Comput. Chem. Eng. 81, 2–21 (2015)
Hefti, M., Marx, D., Joss, L., Mazzotti, M.: Adsorption equilibrium of binary mixtures of carbon dioxide and nitrogen on zeolites ZSM-5 and 13X. Microporous Mesoporous Mater. 215, 215–222 (2015)
Herm, Z.R., Swisher, J.A., Smit, B., Krishna, R., Long, J.R.: Metal-organic frameworks as adsorbents for hydrogen purification and precombustion carbon dioxide capture. J. Am. Chem. Soc. 133, 5664–5667 (2011)
Hiremath, V., Shavi, R., Seo, J.G.: Controlled oxidation state of Ti in MgO-TiO2 composite for CO2 capture. Chem. Eng. J. 308, 177–183 (2017)
Huang, Y., Tao, Y., He, L., Duan, Y., Xiao, J., Li, Z.: Preparation of CuCl@AC with high CO adsorption capacity and selectivity from CO/N2 binary mixture. Adsorption 21(5), 373–381 (2015)
Iruretagoyena, D., Huang, X., Shaffer, M.S.P., Chadwick, D.: Influence of alkali metals (Na, K, and Cs) on CO2 adsorption by layered double oxides supported on graphene oxide. Ind. Eng. Chem. Res. 54(46), 11610–11618 (2015)
Jiao, X., Li, L., Zhao, N., Xiao, F., Wei, W.: Synthesis and low-temperature CO2 capture properties of a novel Mg-Zr solid sorbent. Energy Fuel. 27(9), 5407–5415 (2013)
Kapoor, A., Ritter, J.A., Yang, R.T.: An extended langmuir model for adsorption of gas mixtures on heterogeneous surfaces. Langmuir 6(3), 660–664 (1990)
Koerner, B., Klopatek, J.: Anthropogenic and natural CO2 emission sources in an arid urban environment. Environ. Pollut. 116, S45–S51 (2002)
Kodasma, R., Fermoso, J., Sanna, A.: Li-LSX-zeolite evaluation for post-combustion CO2 capture. Chem. Eng. J. 358, 1351–1362 (2019)
Kim, K., Han, J.W., Lee, K.S., Lee, W.B.: Promoting alkali and alkaline-earth metals on MgO for enhancing CO2 capture by first-principles calculations. Phys. Chem. Chem. Phys: PCCP 16(45), 24818–24823 (2014)
Langmuir, I.: The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40, 1361–1403 (1918)
Lee, S.Y., Park, S.J.: A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 23, 1–11 (2015)
Li, P., Tezel, F.H.: Pure and binary adsorption equilibria of carbon dioxide and nitrogen on silicalite. J. Chem. Eng. Data 53, 2479–2487 (2008)
Li, B., Duan, Y., Luebke, D., Morreale, B.: Advances in CO2 capture technology: a patent review. Appl. Energy 102, 1439–1447 (2013a)
Li, Y.Y., Han, K.K., Lin, W.G., Wan, M.M., Wang, Y., Zhu, J.H.: Fabrication of a new MgO/C sorbent for CO2 capture at elevated temperature. J. Mater. Chem. A 1(41), 12919–12925 (2013b)
Li, D., Tian, Y., Li, L., Li, J., Zhang, H.: Production of highly microporous carbons with large CO2 uptakes at atmospheric pressure by KOH activation of peanut shell char. J. Porous Mater. 22, 1581–1588 (2015)
Lin, Y., Yan, Q., Kong, C., Chen, L.: Polyethyleneimine incorporated metal-Organic frameworks adsorbent for highly selective CO2 capture. Sci. Rep. 3, 1859–1865 (2013)
Ling, Z., Liu, J., Wang, Q., Lin, W., Fang, X., Zhang, Z.: MgCl2·6H2O-Mg(NO3)2·6H2O eutectic/SiO2 composite phase change material with improved thermal reliability and enhanced thermal conductivity. Sol. Energy Mater. Sol. C 172, 195–201 (2017)
Liu, S., Zhang, X., Li, J., Zhao, N., Wei, W., Sun, Y.: Preparation and application of stabilized mesoporous MgO-ZrO2 solid base. Catal. Commun. 9(7), 1527–1532 (2008)
Luebke, R., Eubank, J.F., Cairns, A.J., Belmabkhout, Y., Wojtas, L., Eddaoudi, M.: The unique rht-MOF platform, ideal for pinpointing the functionalization and CO2 adsorption relationship. Chem. Commun. 48, 1455–1457 (2012)
Myers, A.L., Prausnitz, J.M.: Thermodynamics of mixed-gas adsorption. AIChE J. 11(1), 121–127 (1965)
Nikolaidis, G.N., Kikkinides, E.S., Georgiadis, M.C.: A model-based approach for the evaluation of new zeolite 13X-based adsorbents for the efficient post-combustion CO2 capture using P/VSA processes. Chem. Eng. Res. Des. 131, 362–374 (2018)
Olajire, A.A.: CO2 capture and separation technologies for end-of-pipe applications: a review. Energy 35(6), 2610–2628 (2010)
Pires, J., de Carvalho, M.B., Ramoa Ribeiro, F., Derouane, E.G.: Carbon dioxide in Y and ZSM-20 zeolites: adsorption and infrared studies. J. Mol. Catal. 85, 295–303 (1993)
Ramli, N.A.S., Amin, N.A.S.: Fe/HY zeolite as an effective catalyst for levulinic acid production from glucose: characterization and catalytic performance. Appl. Catal. B 163, 487–498 (2015)
Rao, M.B., Sirca, S.: Thermodynamic consistency for binary gas adsorption equilibria. Langmuir 15, 7258–7267 (1999)
Rao, A.B., Rubin, E.S.: A technical, economic and environmental assessment of amine based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 36(20), 4467–4475 (2002)
Razavi, S.S., Hashemianzadeh, S.M., Karimi, H.: Modeling the adsorptive selectivity of carbon nanotubes for effective separation of CO2/N2 mixtures. J. Mol. Model. 17, 1163–1172 (2011)
Regufe, M.J., Ferreira, A.F.P., Loureiro, J.M., Shi, Y., Rodrigues, A., Ribeiro, A.M.: New hybrid composite honeycomb monolith with 13 × zeolite and activated carbon for CO2 capture. Adsorption 24(3), 249–265 (2018)
Rocha, L.A.M., Anne Andreassen, K., Grande, C.A.: Separation of CO2/CH4 using carbon molecular sieve (CMS) at low and high pressure. Chem. Eng. Sci. 164, 148–157 (2017)
Sevilla, M., Fuertes, A.B.: Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ. Sci. 4, 1765–1771 (2011)
Shahkarami, S., Dalai, A.K., Soltan, J.: Enhanced CO2 adsorption using MgO-impregnated activated carbon: Impact of preparation techniques. Ind. Eng. Chem. Res. 55(20), 5955–5956 (2016)
Shao, W., Zhang, L., Li, L., Lee, R.L.: Adsorption of CO2 and N2 on synthesized NaY zeolite at high temperatures. Adsorption 15, 497–505 (2009)
Sips, R.: On the structure of a catalyst surface. J. Chem. Phys. 16, 490–495 (1948)
Song, G., Zhu, X., Chen, R., Liao, Q., Ding, Y.D., Chen, L.: An investigation of CO2 adsorption kinetics on porous magnesium oxide. Chem. Eng. J. 283, 175–183 (2016)
Song, C., Liu, Q., Deng, S., Li, H., Kitamura, Y.: Cryogenic-based CO2 capture technologies: state-of-the-art developments and current challenges. Renew. Sust. Energ. Rev. 101, 265–278 (2019)
Toth, J.: State equations of the solid gas interface layer. Acta Chem. Acad. Hung. 69, 311–317 (1971)
Wang, Q., Luo, J.Z., Zhou, Z.Y., Borgna, A.: CO2 capture by solid adsorbents and their applications: current status and new trends. Energ. Environ. Sci. 4(1), 42–55 (2011)
Wang, C., Li, L., Tang, S., Zhao, X.: Enhanced uptake and selectivity of CO2 Adsorption in a hydrostable metal-organic frameworks via incorporating methylol and methyl groups. ACS Appl. Mater. Interfaces 6, 16932–16940 (2014)
Wilkins, N.S., Rajendran, A.: Measurement of competitive CO2 and N2 adsorption on Zeolite 13X for post-combustion CO2 capture. Adsorption 25, 115–133 (2019)
Wu, Y., Lv, Z., Zhou, X., Peng, J., Tang, Y., Li, Z.: Tuning secondary building unit of Cu-BTC to simultaneously enhance its CO2 selective adsorption and stability under moisture. Chem. Eng. J. 355, 815–821 (2019)
Xiang, S., He, Y., Zhang, Z., Wu, H., Zhou, W., Krishna, R., Chen, B.: Microporous metal-organic framework with potential for carbon dioxide capture at ambient conditions. Nat. Commun. 3, 954–962 (2012)
Yang, S.T., Kim, J., Ahn, W.S.: CO2 adsorption over ion-exchanged zeolite beta with alkali and alkaline earth metal ions. Microporous Mesoporous Mater. 135(1–3), 90–94 (2010)
Zhao, B., Ma, L., Shi, H., Liu, K., Zhang, J.: Calcium precursor from stirring processes at room temperature for controllable preparation of nano-structure CaO sorbents for high temperature CO2 adsorption. J. CO2 Util. 25, 315–322 (2018)
Zukal, A., Pastva, J., Čejka, J.: MgO-modified mesoporous silicas impregnated by potassium carbonate for carbon dioxide adsorption. Microporous Mesoporous Mater. 167, 44–50 (2013)
Acknowledgements
This work has been supported by Natural Science Foundation of Shandong Province (No. ZR2018BB071).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This work does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, F., Wang, S., Chen, G. et al. A facile approach to the fabrication of MgO@Y composite for CO2 capture. Adsorption 26, 701–709 (2020). https://doi.org/10.1007/s10450-019-00147-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-019-00147-w