Abstract
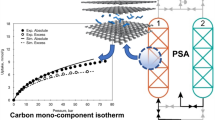
The effect of adsorbent heterogeneity on the performance of an adiabatic pressure swing adsorption (PSA) process for separation of bulk C2H4 from an inert gas (helium) using the BPL carbon as the adsorbent was numerically estimated employing a detailed mathematical model for the process. The heterogeneous Toth model was used to describe the equilibrium adsorption isotherms for C2H4 and a linear driving force model was used to describe the adsorbate mass transfer rate in the simulation. The variations in the isosteric heat of adsorption of C2H4 with adsorbate loading was included in the simulation. The study indicates that adsorbent heterogeneity negatively affects the key process performance variables by increasing the bed size factor and reducing the helium recovery. Actual performance data for a PSA process using a bench or pilot scale unit is required to reliably estimate the complexity introduced by adsorbent heterogeneity.




Similar content being viewed by others
References
Cao, D.V., Sircar, S.: Temperature dependence of isosteric heat of adsorption. Adsorpt. Sci. Technol. 19(10), 887–894 (2001)
Chai, S.W., Kothare, M.V., Sircar, S.: Rapid pressure swing adsorption for reduction of bed size factor of a medical oxygen concentrator. Ind. Eng. Chem. Res. 50(14), 8703–8710 (2011)
Ergun, S.: Fluid flow through packed columns. Chem. Eng. Prog. 48(2), 89–94 (1952)
Gregg, S.J., Sing, K.S.W.: Adsorption, Surface Area, and Porosity. Academic Press, London (1967)
Haghpanah, R., Mujumdar, A., Nilam, R., Rajendran, A., Farooq, S., Karimi, I.A., Amanullah, M.: Multiobjective optimization of a four-step adsorption process for post-combustion CO2 capture via finite volume simulation. Ind. Eng. Chem. Res. 52(11), 4249–4265 (2013)
Hartzog, D.G., Sircar, S.: Sensitivity of PSA process performance to input variables. Adsorption 1(2), 133–151 (1995)
Rama Rao, V., Kothare, M.V., Sircar, S.: Novel design and performance of a medical oxygen concentrator using a rapid pressure swing adsorption concept. AIChE J. 60(9), 3330–3335 (2014)
Rama Rao, V., Kothare, M.V., Sircar, S.: Anatomy of a pressure swing adsorption process performance. AIChE J. 61(6), 2008–2015 (2015)
Reich, R., Ziegler, W.T., Rogers, K.A.: Adsorption of methane, ethane, and ethylene gases and their binary and ternary mixtures and carbon dioxide on activated carbon at 212–301 K and pressures to 35 atmospheres. Ind. Eng. Chem. Process Des. Dev. 19(3), 336–344 (1980)
Ruthven, D.M.: Principles of Adsorption and Adsorption Processes. Wiley, New York (1984)
Sircar, S.: Isosteric heats of multicomponent gas adsorption on heterogeneous adsorbents. Langmuir 7(12), 3065–3069 (1991)
Sircar, S.: Chapter 59-Adsorption. In: Dort, R.C. (ed.) The Engineering Handbook, pp. 604–617. CRC Press, Boca Raton (1996)
Sircar, S., Hufton, J.R.: Why does linear driving force model for adsorption kinetics work? Adsorption 6(2), 137–147 (2000)
Toth, J.: State equations of the solid gas interface layer. Acta Chem. Acad. Hung. 69, 311–317 (1971)
Valenzuela, D.P., Myers, A.L.: Adsorption equilibrium data handbook. Prentice-Hall, Englewood Cliffs, New Jersey (1989)
Wakao, N., Kaguei, S., Funazkri, T.: Effect of fluid dispersion coefficients on particle to fluid heat transfer coefficients in packed beds. Chem. Eng. Sci. 34(3), 325–336 (1979)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vemula, R.R., Sircar, S. Effect of adsorbent heterogeneity on performance of a PSA process for bulk gas separation: a parametric simulation. Adsorption 24, 415–422 (2018). https://doi.org/10.1007/s10450-018-9947-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-018-9947-0