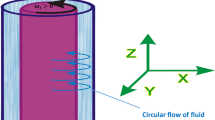
Abstract
One of the main ocular diseases is age-related macular degeneration, actually treated with antibodies injections into the eye. This problem has been faced by computational approaches, taking into account either the influence of the tissues surrounding the vitreous, or the saccades. The aim of this work is to propose a combined fluid dynamic model of the vitreous chamber that analyses the impact of the saccades on the fluid dynamic mechanisms. The ocular vitreous humor was modeled considering liquefaction occurring in presence of age-related macular degeneration. We identified two kinds of boundary conditions, one related to the physiological environment outside the chamber, and one related to the saccades. The scleral hydraulic conductivity was evaluated by means of experimental permeability tests. An exponential decay was used to describe the trend of the scleral hydraulic conductivity with the acting pressure drop. The streamline analysis shows two main stagnant regions on the equatorial plane and peculiar fluid dynamics in absence of saccades. This study demonstrates the major role played by the saccades in determining the fluid dynamic mechanisms inside the vitreous chamber of the eye and represents a powerful tool to investigate vitreous dynamics and its relation to clinical issues.







Similar content being viewed by others
Abbreviations
- AMD:
-
Age-related macular degeneration
- VEGF:
-
Vascular endothelial growth factors
- PIV:
-
Particle image velocimetry
- CFD:
-
Computational fluid dynamics
- RCS:
-
Retina–choroid–sclera
- IOP:
-
Intraocular pressure
- HC:
-
Hydraulic conductivity
References
Abouali, O., A. Modareszadeh, A. Ghaffariyeh, and J. Tu. Numerical simulation of the fluid dynamics in vitreous cavity due to saccadic eye movement. Med. Eng. Phys. 34:681–692, 2012.
Argento, A., W. Kim, F. W. Rozsa, K. L. DeBolt, S. Zikanova, and J. R. Richards. Shear behavior of bovine scleral tissue. J. Biomech. Eng. 136:071011, 2014.
Balachandran, R. K., and V. H. Barocas. Computer modeling of drug delivery to the posterior eye: effect of active transport and loss to choroidal blood flow. Pharm. Res. 25:2685–2696, 2008.
Balachandran, R. K., and V. H. Barocas. Contribution of saccadic motion to intravitreal drug transport: theoretical analysis. Pharm. Res. 28:1049–1064, 2011.
Balazs, E. A., and M. T. Flood. Age-related changes in the physical and chemical structure of human vitreous. Third International Congress of Eye Research, 1978.
Becker, W. The neurobiology of saccadic eye movements. Metrics. Rev. Oculomot. Res. 3:13, 1989.
Bhisitkul, R. B. Anticipation for enzymatic vitreolysis. Br. J. Ophthalmol. 85:1–2, 2001.
Bonfiglio, A., A. Lagazzo, R. Repetto, and A. Stocchino. An experimental model of vitreous motion induced by eye rotations. Eye Vis. 2:10, 2015.
Chan, C. M., J. H. Yu, L. J. Chen, C. H. Huang, C. T. Lee, T. C. Lin, and D. Z. Liu. Posterior pole retinal thickness measurements by the retinal thickness analyzer in healthy Chinese subjects. Retina 26:176–181, 2006.
Cima, M. J., H. Lee, K. Daniel, L. M. Tanenbaum, A. Mantzavinou, K. C. Spencer, Q. Ong, J. C. Sy, J. Santini, C. M. Schoellhammer, D. Blankschtein, and R. S. Langer. Single compartment drug delivery. J. Control Release 190:157–171, 2014.
David, T., S. Smye, T. Dabbs, and T. James. A model for the fluid motion of vitreous humour of the human eye during saccadic movement. Phys. Med. Biol. 43:1385–1399, 1998.
Fatt, I., and B. O. Hedbys. Flow of water in the sclera. Exp. Eye Res. 10:243–249, 1970.
Ferrara, N. Vascular endothelial growth factor and age-related macular degeneration: from basic science to therapy. Nat. Publ. Gr. 16:1107–1111, 2010.
Ferrara, N., H. P. Gerber, and J. LeCouter. The biology of VEGF and its receptors. Nat Med 9:669–676, 2003.
Friedrich, S., Y.-L. Cheng, and B. Saville. Drug distribution in the vitreous humor of the human eye: the effects of intravitreal injection position and volume. Curr. Eye Res. 16:663–669, 1997.
Haghjou, N., M. J. Abdekhodaie, Y. L. Cheng, and M. Saadatmand. Computer modeling of drug distribution after intravitreal administration. World Acad. Sci. Eng. Technol. 77:706–716, 2011.
Ikuno, Y., K. Kawaguchi, T. Nouchi, and Y. Yasuno. Choroidal thickness in healthy Japanese subjects. Investig. Opthalmol. Vis. Sci. 51:2173, 2010.
Jackson, T. L., A. Hussain, A. Hodgetts, A. M. S. Morley, J. Hillenkamp, P. M. Sullivan, and J. Marshall. Human scleral hydraulic conductivity: age-related changes, topographical variation, and potential scleral outflow facility. Investig. Ophthalmol. Vis. Sci. 47:4942–4946, 2006.
Lai, W. M., V. C. Mow, and V. Roth. Effects of nonlinear strain-dependent permeability and rate of compression on the stress behavior of articular cartilage. J. Biomech. Eng. 103:61–66, 1981.
Lee, B., M. Litt, and G. Buchsbaum. Rheology of the vitreous body. Part I: Viscoelasticity of human vitreous. Biorheology 29:521–533, 1992.
Loudon, C., and A. Tordesillas. The use of the dimensionless Womersley number to characterize the unsteady nature of internal flow. J. Theor. Biol. 191:63–78, 1998.
Missel, P. J. Hydraulic flow and vascular clearance influences on intravitreal drug delivery. Pharm. Res. 19:1636–1647, 2002.
Modareszadeh, A., O. Abouali, A. Ghaffarieh, and G. Ahmadi. Saccade movements effect on the intravitreal drug delivery in vitreous substitutes: a numerical study. Biomech. Model. Mechanobiol. 12:281–290, 2013.
Repetto, R., J. H. Siggers, and A. Stocchino. Mathematical model of flow in the vitreous humor induced by saccadic eye rotations: effect of geometry. Biomech. Model. Mechanobiol. 9:65–76, 2010.
Repetto, R., A. Stocchino, and C. Cafferata. Experimental investigation of vitreous humour motion within a human eye model. Phys. Med. Biol. 50:4729–4743, 2005.
Romano, M. R., J. L. Vallejo-Garcia, V. Romano, M. Angi, P. Vinciguerra, and C. Costagliola. Thermodynamics of vitreoretinal surgery. Curr. Eye Res. 38:371–374, 2013.
Rosenfeld, P. J., D. M. Brown, J. S. Heier, D. S. Boyer, P. K. Kaiser, C. Y. Chung, and R. Y. Kim. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 355:1419–1431, 2006.
Rosenfeld, P. J., S. D. Schwartz, M. S. Blumenkranz, J. W. Miller, J. A. Haller, J. D. Reimann, W. L. Greene, and N. Shams. Maximum tolerated dose of a humanized anti-vascular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmology 112:1048.e4–1053.e4, 2005.
Schwartz, S. G., I. U. Scott, H. W. Flynn, and M. W. Stewart. Drug delivery techniques for treating age-related macular degeneration. Expert Opin. Drug Deliv. 11:61–68, 2014.
Sekuler, R., D. Kline, and K. Dismukes. Aging and visual function of military pilots: a review. DTIC Document, 1982.
Stay, M. S., J. Xu, T. W. Randolph, and V. H. Barocas. Computer simulation of convective and diffusive transport of controlled-release drugs in the vitreous humor. Pharm. Res. 20:96–102, 2003.
Stocchino, A., R. Repetto, and C. Cafferata. Eye rotation induced dynamics of a Newtonian fluid within the vitreous cavity: the effect of the chamber shape. Phys. Med. Biol. 52:2021–2034, 2007.
Stocchino, A., R. Repetto, and J. H. Siggers. Mixing processes in the vitreous chamber induced by eye rotations. Phys. Med. Biol. 55:453–467, 2010.
Vaiano, A. S., E. Coronado Quitllet, G. Zinzanella, G. De Benedetti, and G. Caramello. Ultrasound measurements of the distance between limbus and retinal break in eyes with media opacities. Retina 37:1400–1406, 2017.
Vurgese, S., S. Panda-Jonas, and J. B. Jonas. Scleral thickness in human eyes. PLoS ONE 7:e29692, 2012.
Xu, J., J. J. Heys, V. H. Barocas, and T. W. Randolph. Permeability and diffusion in vitreous humor: implications for drug delivery. Pharm. Res. 17:664–669, 2000.
Zetterberg, M. Age-related eye disease and gender. Maturitas 83:19–26, 2016.
Acknowledgments
This study was funded by the Italian Ophthalmological Society.
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Estefanía Peña oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.


Rights and permissions
About this article
Cite this article
Ferroni, M., Cereda, M.G. & Boschetti, F. A Combined Approach for the Analysis of Ocular Fluid Dynamics in the Presence of Saccadic Movements. Ann Biomed Eng 46, 2091–2101 (2018). https://doi.org/10.1007/s10439-018-02110-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-018-02110-2