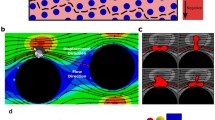
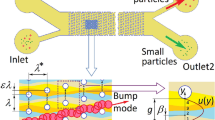
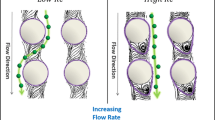
Abstract
The deterministic lateral displacement (DLD) is an important method used to sort particles and cells of different sizes. In this paper, the flexible cell sorting with the DLD method is studied by using a numerical model based on the immersed boundary-lattice Boltzmann method (IB-LBM). In this model, the fluid motion is solved by the LBM, and the cell membrane–fluid interaction is modeled with the LBM. The proposed model is validated by simulating the rigid particle sorted with the DLD method, and the results are found in good agreement with those measured in experiments. We first study the effect of flexibility on a single cell and multiple cells continuously going through a DLD device. It is found that the cell flexibility can significantly affect the cell path, which means the flexibility could have significant effects on the continuous cell sorting by the DLD method. The sorting characteristics of white blood cells and red blood cells are further studied by varying the spatial distribution of cylinder arrays and the initial cell–cell distance. The numerical results indicate that a well concentrated cell sorting can be obtained under a proper arrangement of cylinder arrays and a large enough initial cell–cell distance.









Similar content being viewed by others
References
Shevkoplyas, S.S., Yoshida, T., Munn, L.L., et al.: Biomimetic autoseparation of leukocytes from whole blood in a microfluidic device. Anal. Chem. 77, 933–937 (2005)
VanDelinder, V., Groisman, A.: Separation of plasma from whole human blood in a continuous cross-flow in a molded microfluidic device. Anal. Chem. 78, 3765–3771 (2006)
Nagrath, S., Sequist, L.V., Maheswaran, S., et al.: Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450, 1235–1239 (2007)
Dharmasiri, U., Witek, M.A., Adams, A.A., et al.: Microsystems for the capture of low-abundance cells. Annu. Rev. Anal. Chem. 3, 409–431 (2010)
Bhagat, A.A.S., Hou, H.W., Li, L.D., et al.: Pinched flow coupled shear-modulated inertial microfluidics for high-throughput rare blood cell separation. Lab Chip 11, 1870–1878 (2011)
Inglis, D.W., Riehn, R., Austin, R.H., et al.: Continuous microfluidic immunomagnetic cell separation. Appl. Phys. Lett. 85, 5093–5095 (2004)
Crowley, T.A., Pizziconi, V.: Isolation of plasma from whole blood using planar microfilters for lab-on-a-chip applications. Lab Chip 5, 922–929 (2005)
Huh, D., Gu, W., Kamotani, Y., et al.: Microfluidics for flow cytometric analysis of cells and particles. Physiol. Meas. 26, R73–R98 (2005)
Han, K.H., Frazier, A.B.: Diamagnetic capture mode magnetophoretic microseparator for blood cells. J. Microelectromech. Syst. 14, 1422–1431 (2005)
Han, K.H., Frazier, A.B.: Paramagnetic capture mode magnetophoretic microseparator for high efficiency blood cell separations. Lab Chip 6, 265–273 (2006)
Wang, M.M., Tu, E., Raymond, D.E., et al.: Microfluidic sorting of mammalian cells by optical force switching. Nat. Biotechnol. 23, 83–87 (2005)
Murata, M., Okamoto, Y., Park, Y.S., et al.: Cell separation by the combination of microfluidics and optical trapping force on a microchip. Anal. Bioanal. Chem. 394, 277–283 (2009)
Wilding, P., Kricka, L.J., Cheng, J., et al.: Integrated cell isolation and polymerase chain reaction analysis using silicon microfilter chambers. Anal. Chem. 257, 95–100 (1998)
Mohamed, H., McCurdy, L.D., Szarowski, D.H., et al.: Development of a rare cell fractionation device: application for cancer detection. IEEE Trans. Nanobiosci. 3, 251–256 (2004)
Pamme, N.: Continuous flow separations in microfluidic devices. Lab Chip 7, 1644–1659 (2007)
Tsutsui, H., Ho, C.M.: Cell separation by non-inertial force fields in microfluidic systems. Mech. Res. Commun. 36, 92–103 (2009)
Yang, S., Undar, A., Zahn, J.D.: A microfluidic device for continuous, real time blood plasma separation. Lab Chip 6, 871–880 (2006)
Hou, H.W., Bhagat, A.A.S., Lee, W.C., et al.: Microfluidic devices for blood fractionation. Micromachines 2, 319–343 (2011)
Choi, S., Song, S., Choi, C., et al.: Continuous blood cell separation by hydrophoretic filtration. Lab Chip 7, 1532–1538 (2007)
Bhagat, A.A.S., Bow, H., Hou, H.W., et al.: Microfluidics for cell separation. Med. Biol. Eng. Comput. 48, 999–1014 (2010)
Gossett, D.R., Weaver, W.M., Mach, A.J., et al.: Label-free cell separation and sorting in microfluidic systems. Anal. Bioanal. Chem. 397, 3249–3267 (2010)
Andersen, K.B., Levinsen, S., Svendsen, W.E., et al.: A generalized theoretical model for “continuous particle separation in a microchannel having asymmetrically arranged multiple branches”. Lab Chip 9, 1638–1639 (2009)
Huang, L.R., Cox, E.C., Austin, R.H., et al.: Continuous particle separation through deterministic lateral displacement. Science 304, 987–990 (2004)
McGrath, J., Jimenez, M., Bridle, H.: Deterministic lateral displacement for particle separation: a review. Lab Chip 14, 4139–4158 (2014)
Davis, J.A., Inglis, D.W., Morton, K.J., et al.: Deterministic hydrodynamics: taking blood apart. Proc. Natl Acad. Sci. U.S.A. 103, 14779–14784 (2006)
Green, J.V., Radisic, M., Murthy, S.K.: Deterministic lateral displacement as a means to enrich large cells for tissue engineering. Anal. Chem. 81, 9178–9182 (2009)
Morton, K.J., Loutherback, K., Inglis, D.W., et al.: Hydrodynamic metamaterials: microfabricated arrays to steer, refract, and focus streams of biomaterials. Proc. Natl Acad. Sci. U.S.A. 105, 7434–7438 (2008)
Morton, K.J., Loutherback, K., Inglis, D.W., et al.: Crossing microfluidic streamlines to lyse, label and wash cells. Lab Chip 8, 1448–1453 (2008)
Zhang, J., Johnson, P.C., Popel, A.S.: Red blood cell aggregation and dissociation in shear flows simulated by lattice Boltzmann method. J. Biomech. 41, 47–55 (2008)
Xu, Y.Q., Tang, X.Y., Tian, F.B., et al.: IB-LBM simulation of the haemocyte dynamics in a stenotic capillary. Comput. Methods Biomech. 17, 978–985 (2014)
Wei, Q., Xu, Y.Q., Tian, F.B., et al.: IB-LBM simulation on blood cell sorting with a micro-fence structure. Biomed. Mater. Eng. 24, 475–481 (2014)
Ma, J.T., Xu, Y.Q., Tian, F.B., et al.: IB-LBM study on cell sorting by pinched flow fractionation. Biomed. Mater. Eng. 24, 2547–2554 (2014)
Krueger, T., Holmes, D., Coveney, P.V.: Deformability-based red blood cell separation in deterministic lateral displacement devices—a simulation study. Biomicrofluidics 8, 054114 (2014)
Chang, C.B., Huang, W.X., Lee, K.H., et al.: Optical separation of ellipsoidal particles in a uniform flow. Phys. Fluids 26, 062001 (2014)
Chang, C.B., Huang, W.X., Sung, H.J.: Cross-type optical separation of elastic oblate capsules in a uniform flow. J. Appl. Phys. 117, 034701 (2015)
Chang, C.B., Huang, W.X., Sung, H.J.: Migration of elastic capsules by an optical force in a uniform flow. Procedia IUTAM 16, 50–59 (2015)
Quek, R., Le, D.V., Chiam, K.H.: Separation of deformable particles in deterministic lateral displacement devices. Phys. Rev. E 83, 05630 (2011)
Xu, Y.Q., Tian, F.B., Deng, Y.L.: An efficient red blood cell model in the frame of IB-LBM and its application. Int. J. Biomath. 6, 1250061 (2013)
Qian, Y.H., D’Humières, D., Lallemand, P.: Lattice BGK models for Navier–Stokes equation. Europhys. Lett. 17, 479–484 (1992)
Tian, F.B., Luo, H., Zhu, L., et al.: An efficient immersed boundary-lattice Boltzmann method for the hydrodynamic interaction of elastic filaments. J. Comput. Phys. 230, 7266–7283 (2011)
Guo, Z., Zheng, C., Shi, B.: Discrete lattice effects on the forcing term in the lattice Boltzmann method. Phys. Rev. E 65, 046308 (2002)
Peskin, C.S.: The immersed boundary method. Acta Numer. 11, 479–517 (2002)
Guo, Z.L., Zheng, C.G., Shi, B.C.: Non-equilibrium extrapolation method for velocity and pressure boundary conditions in the lattice Boltzmann method. Chin. Phys. 11, 366–374 (2002)
Zhang, J., Johnson, P.C., Popel, A.: An immersed boundary lattice Boltzmann approach to simulate deformable liquid capsules and its application to microscopic blood flows. Phys. Biol. 4, 285–295 (2007)
Pan, T.W., Wang, T.: Dynamical simulation of red blood cell rheology in microvessels. Int. J. Numer. Anal. Mod. 6, 455–473 (2009)
Sun, C., Munn, L.L.: Particulate nature of blood determines macroscopic rheology: a 2-D lattice Boltzmann analysis. Biophys. J. 88, 1635–1645 (2005)
Inglis, D.W., Davis, J.A., Austin, R.H., et al.: Critical particle size for fractionation by deterministic lateral displacement. Lab Chip 6, 655–658 (2006)
Acknowledgments
This project was supported by the National Natural Science Foundation of China (Grant 81301291), the Beijing Higher Education Young Elite Teacher Project (Grant YETP1208), and UNSW Special Research Grants Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, Q., Xu, YQ., Tang, XY. et al. An IB-LBM study of continuous cell sorting in deterministic lateral displacement arrays. Acta Mech. Sin. 32, 1023–1030 (2016). https://doi.org/10.1007/s10409-016-0566-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10409-016-0566-2