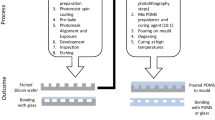
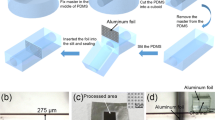
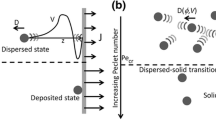
Abstract
The integration of porous membranes with microfluidic devices allows a simple but high-throughput mass transport control for numerous microfluidic applications, such as single-cell separation, sample analysis, and purification. In this study, we demonstrate a novel integration process of porous membranes into microfluidic devices by applying a magnetic field and hydrodynamically stabilizing them. This new approach simplifies the integration process by removing physicochemical bonding between membranes and microfluidic devices, but overcomes many practical issues observed in current methods, such as device leakage, membrane replacement, and membrane material selection. More importantly, our approach allows us to install membranes with diverse physicochemical features and spatial configurations into a single microfluidic device. This additional ability can significantly improve its performance and capability in applications. Finally, we successfully demonstrate the utilization of our membrane device for simple particle separation.




Similar content being viewed by others
References
Brown AJ et al (2014) Interfacial microfluidic processing of metal-organic framework hollow fiber membranes. Science 345:72–75
Chen X, Shen J (2017) Review of membranes in microfluidics. J Chem Technol Biotechnol 92:271–282
Chen MB, Srigunapalan S, Wheeler AR, Simmons CA (2013) A 3D microfluidic platform incorporating methacrylated gelatin hydrogels to study physiological cardiovascular cell–cell interactions. Lab Chip 13:2591–2598
Chueh B-H et al (2007) Leakage-free bonding of porous membranes into layered microfluidic array systems. Anal Chem 79:3504–3508
de Jong J, Lammertink RG, Wessling M (2006) Membranes and microfluidics: a review. Lab Chip 6:1125–1139
Dendukuri D, Gu SS, Pregibon DC, Hatton TA, Doyle PS (2007) Stop-flow lithography in a microfluidic device. Lab Chip 7:818–828
Eddings MA, Johnson MA, Gale BK (2008) Determining the optimal PDMS–PDMS bonding technique for microfluidic devices. J Micromech Microeng 18:067001
Epshteyn AA et al (2011) Membrane-integrated microfluidic device for high-resolution live cell imaging. Biomicrofluidics 5:46501–465016
Frohlich EM et al (2013) Topographically-patterned porous membranes in a microfluidic device as an in vitro model of renal reabsorptive barriers. Lab Chip 13:2311–2319
Gao D, Liu H, Lin J-M, Wang Y, Jiang Y (2013) Characterization of drug permeability in Caco-2 monolayers by mass spectrometry on a membrane-based microfluidic device. Lab Chip 13:978–985
Girault H (2002) Integration of a membrane-based desalting step in a microfabricated disposable polymer injector for mass spectrometric protein analysis. Electrophoresis 23:3583–3588
Hisamoto H et al (2003) Chemicofunctional membrane for integrated chemical processes on a microchip. Anal Chem 75:350–354
Ho WF, Lim KM, Yang KL (2016a) In situ formation of leak-free polyethylene glycol (PEG) membranes in microfluidic fuel cells. Lab Chip 16:4725–4731
Ho KK, Lee LM, Liu AP (2016b) Mechanically activated artificial cell by using microfluidics. Sci Rep 6:32912
Hu C et al (2016) A one-step strategy for ultra-fast and low-cost mass production of plastic membrane microfluidic chips. Lab Chip 16:3909–3918
Huh D et al (2010) Reconstituting organ-level lung functions on a chip. Science 328:1662–1668
Jain T, Guerrero RJ, Aguilar CA, Karnik R (2013) Integration of solid-state nanopores in microfluidic networks via transfer printing of suspended membranes. Anal Chem 85:3871–3878
Li M, Humayun M, Kozinski JA, Hwang DK (2014a) Functional polymer sheet patterning using microfluidics. Langmuir ACS J Surf Colloids 30:8637–8644
Li X, Chen W, Liu G, Lu W, Fu J (2014b) Continuous-flow microfluidic blood cell sorting for unprocessed whole blood using surface-micromachined microfiltration membranes. Lab Chip 14:2565–2575
Lion N, Gellon J-O, Jensen H, Girault HH (2003) On-chip protein sample desalting and preparation for direct coupling with electrospray ionization mass spectrometry. J Chromatogr A 1003:11–19
Lu JC, Liao WH, Tung YC (2012) Magnet-assisted device-level alignment for the fabrication of membrane-sandwiched polydimethylsiloxane microfluidic devices. J Micromech Microeng 22:075006
Lusianti RE, Higgins AZ (2014) Continuous removal of glycerol from frozen-thawed red blood cells in a microfluidic membrane device. Biomicrofluidics 8:054124
Maruyama T et al (2004) Liquid membrane operations in a microfluidic device for selective separation of metal ions. Anal Chem 76:4495–4500
Metz S, Trautmann C, Bertsch A, Renaud P (2003) Polyimide microfluidic devices with integrated nanoporous filtration areas manufactured by micromachining and ion track technology. J Micromech Microeng 14:324
Prechtl C, Kraut M, Franzreb M, Brenner-Weiß G, Dittmeyer R (2017) Membrane-supported multichannel microfluidic solvent extraction system. Chem Eng Technol 40:670–677
Wang P-C, Gao J, Lee CS (2002) High-resolution chiral separation using microfluidics-based membrane chromatography. J Chromatogr A 942:115–122
Wang C, Gao X, Mawatari K, Kitamori T (2017) Clogging-free irreversible bonding of polycarbonate membranes to glass microfluidic devices. J Electrochem Soc 164:B3087–B3090
Wei H et al (2011) Particle sorting using a porous membrane in a microfluidic device. Lab Chip 11:238–245
Xia Y, Whitesides GM (1998) Soft lithography. Annu Rev Mater Sci 28:153–184
Xu J, Vaillant R, Attinger D (2010) Use of a porous membrane for gas bubble removal in microfluidic channels: physical mechanisms and design criteria. Microfluid Nanofluid 9:765–772
Yang HY et al (2013) Carbon nanotube membranes with ultrahigh specific adsorption capacity for water desalination and purification. Nat Commun 4:2220
Zheng S et al (2007) Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J Chromatogr A 1162:154–161
Acknowledgements
The authors acknowledge the Natural Sciences and Engineering Research Council of Canada (Discovery Grant RGPIN-2017-04489) and Canada Research Chairs for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (AVI 1737 KB)
Supplementary material 4 (AVI 1070 KB)
Supplementary material 5 (AVI 166557 KB)
Supplementary material 6 (AVI 309 KB)
Supplementary material 7 (AVI 11882 KB)
Rights and permissions
About this article
Cite this article
Han, S., Hwang, D.K. No more bonding, no more clamping, magnetically assisted membrane integration in microfluidic devices. Microfluid Nanofluid 22, 107 (2018). https://doi.org/10.1007/s10404-018-2127-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-018-2127-4