Abstract
Purpose
Atropine eye drops prevent the progression of myopia, but their use has not been tested in the Japanese schoolchildren population. Here, we evaluate the efficacy and safety of 0.01% atropine eye drops for myopia control in Japanese children.
Study design
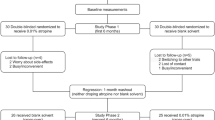
Multicenter (7 university hospitals), randomized, double-masked, placebo-controlled trial.
Methods
Participants were 171 Japanese schoolchildren aged 6 to 12 years, with progressive myopia, spherical equivalence (SE) of −1.00 to −6.00 diopters (D), and astigmatism of ≤1.5 D. They were randomized to receive either 0.01% atropine (n=85) or placebo (n=86) eye drops once nightly OU for 24 months. Primary and secondary efficacy endpoints were changes in SE and axial length (AL), respectively, from baseline to month 24.
Results
Data from 168 subjects were analyzed. At month 24, compliance was similar in both groups (atropine: 83.3%; placebo: 85.7%). The least squares mean change in SE and AL from baseline were, respectively, −1.26 D (95% confidence interval [CI]: −1.35, −1.17) and 0.63 mm (0.59, 0.67) for atropine and −1.48 D (− 1.57, −1.39) and 0.77 mm (0.73, 0.81) for placebo. Inter-group differences were 0.22 D (95% CI: 0.09, 0.35; P < 0.001) for SE and − 0.14 mm (−0.20, −0.08; P < 0.001) for AL. Three patients experienced mild allergic conjunctivitis side effects, with no inter-group difference in incidence (atropine: 2.4%; 2/84 patients; placebo: 1.4%; 1/84 patients).
Conclusion
With good compliance, 0.01% atropine is effective and safe for preventing the progression of childhood myopia.





Similar content being viewed by others
References
Saw SM, Matsumura S, Hoang QV. Prevention and management of myopia and myopic pathology. Invest Ophthalmol Vis Sci. 2019;60:488–99.
Rudnicka AR, Kapetanakis VV, Wathern AK, Logan NS, Gilmartin B, Whincup PH, et al. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. Br J Ophthalmol. 2016;100:882–90.
Ministry of Education, Culture, Sports, Science and Technology. Annual Report of School Health Statistics Research. 2018. http://www.mext.go.jp/b_menu/toukei/chousa05/hoken/kekka/k_detail/1411711.htm. Accessed 27 Mar 2019.
Takashima T, Yokoyama T, Futagami S, Ohno-Matsui K, Tanaka H, Tokoro T, et al. The quality of life in patients with pathologic myopia. Jpn J Ophthalmol. 2001;45:84–92.
Asakuma T, Yasuda M, Ninomiya T, Noda Y, Arakawa S, Hashimoto S, et al. Prevalence and risk factors for myopic retinopathy in a Japanese population: the Hisayama Study. Ophthalmology. 2012;119:1760–5.
Gong Q, Janowski M, Luo M, Wei H, Chen B, Yang G, et al. Efficacy and adverse effects of atropine in childhood myopia: a meta-analysis. JAMA Ophthalmol. 2017;135:624–30.
Wu PC, Chuang MN, Choi J, Chen H, Wu G, Ohno-Matsui K, et al. Update in myopia and treatment strategy of atropine use in myopia control. Eye (Lond). 2019;33:3–13.
Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113:2285–91.
Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012;119:347–54.
Clark TY, Clark RA. Atropine 0.01% eyedrops significantly reduce the progression of childhood myopia. J Ocul Pharmacol Ther. 2015;31:541–5.
Yam JC, Jiang Y, Tang SM, Law AKP, Chan JJ, Wong E, et al. Low-concentration atropine for myopia progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126:113–24.
Yam JC, Li FF, Zhang X, Tang SM, Yip B, Kam KW, et al. Two-year clinical trial of the Low-concentration Atropine for Myopia Progression (LAMP) study: phase 2 report. Ophthalmology. 2020;127:910–9.
Hasebe S, Ohtsuki H, Nonaka T, Nakatsuka C, Miyata M, Hamasaki I, et al. Effect of progressive addition lenses on myopia progression in Japanese children: a prospective, randomized, double-masked, crossover trial. Invest Ophthalmol Vis Sci. 2008;49:2781–9.
Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol. 2014;157:451.e1-7.e1.
Kinoshita S. Pathogenesis and treatment of accommodative disturbance. Nippon Ganka Gakkai Zasshi. 1994;98:1256–68 ((in Japanese)).
Stone RA, Pardue MT, Iuvone PM, Khurana TS. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res. 2013;114:35–47.
Vagge A, Ferro Desideri L, Nucci P, Serafino M, Giannaccare G, Traverso CE. Prevention of progression in myopia: A systematic review. Diseases. 2018;6:92.
McBrien NA, Moghaddam HO, Reeder AP. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Invest Ophthalmol Vis Sci. 1993;34:205–15.
McBrien NA, Jobling AI, Truong HT, Cottriall CL, Gentle A. Expression of muscarinic receptor subtypes in tree shrew ocular tissues and their regulation during the development of myopia. Mol Vis. 2009;15:464–75.
Cottriall CL, McBrien NA. The M1 muscarinic antagonist pirenzepine reduces myopia and eye enlargement in the tree shrew. Invest Ophthalmol Vis Sci. 1996;37:1368–79.
Siatkowski RM, Cotter SA, Crockett RS, Miller JM, Novack GD, Zadnik K, U.S. Pirenzepine Study Group. Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. J AAPOS. 2008;12:332–9.
Nishiyama Y, Moriyama M, Fukamachi M, Uchida A, Miyaushiro H, Kurata A, et al. Side effects of low dose atropine. Nippon Ganka Gakkai Zasshi. 2015;119:812–6 (in Japanese).
Chia A, Lu QS, Tan D. Five-year clinical trial on atropine for the treatment of myopia 2: Myopia control with atropine 0.01% eyedrops. Ophthalmology. 2016;123:391–9.
Acknowledgements
The authors wish to thank Mr. John Bush and Mr. Takahiko Murata for editing the manuscript. This study was supported by Eye-Lens Pte., Ltd., Singapore. The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
O. Hieda, None; T. Hiraoka, None; T. Fujikado, None; S. Ishiko, None; S. Hasebe, None; H. Torii, Honorarium for lecturing (Santen, Otsuka, HOYA, Alcon, ROHTO), Remuneration (Tsubota Laboratory), Honorarium (MediProduce); H. Takahashi, None; Y. Nakamura, None; C. Sotozono, None; T. Oshika, None; T. Morimoto, None; K. Nishida, None; N. Nishikawa, None; Y.S. Song, None; T. Tokutake, None; Y. Nishi, None; Y. Shigeno, None; T. Kurihara, Grant (Tsubota Laboratory, Fuji Xerox, KIRIN, Kowa, Santen, SEED), Grant, Honorarium for lecturing (Novartis, ROHTO), Honorarium for lecturing (Bayer); K. Negishi, Grant, Honorarium for lecturing (Alcon, Universal View, Abbott, Kowa, Santen, Hitachi, Senju, TOMEY), Grant (Fuji Xerox, Bausch & Lomb), Grant, Honorarium for lecturing, Non-Financial support (HOYA), Consultant fee (NIDEK), Honorarium for lecturing (Otsuka, ZEISS, santec); K. Tsubota, Grant (Tsubota Laboratory, ROHTO), Tsubota Laboratory and the author own the patent for Myopia Prevention Article; M. Ono, None; T. Nakai, None; D. Tan, Consultant fee (Eye-Lens Pte); S. Tanaka, Consultant fee (Boehringer Ingelheim), Non-financial support (AstraZeneca, Taiho, Ono, JMDC, DeNA Life Science), Honorarium for lecturing (Amgen); S. Kinoshita, Grant, Honorarium for lecturing, Consulting (Santen, Otsuka), Honorarium for Lecturing, Consulting (Senju, Alcon), Honorarium for Lecturing (Kowa, Johnson & Johnson), Grant (HOYA, CorneaGen), Honorarium for Consulting (Novartis).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Shigeru Kinoshita
Supplementary Information
Below is the link to the electronic supplementary material.
10384_2021_822_MOESM1_ESM.docx
Online Resource 1 Analysis of the Change of Spherical Equivalent and Axial Length from at Enrollment using Mixed Models (DOCX 17 KB)
10384_2021_822_MOESM2_ESM.docx
Online Resource 2 Primary Analysis of Spherical Equivalent and Axial Length using Mixed Models for Per Protocol Set (DOCX 19 KB)
About this article
Cite this article
Hieda, O., Hiraoka, T., Fujikado, T. et al. Efficacy and safety of 0.01% atropine for prevention of childhood myopia in a 2-year randomized placebo-controlled study. Jpn J Ophthalmol 65, 315–325 (2021). https://doi.org/10.1007/s10384-021-00822-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-021-00822-y