Abstract
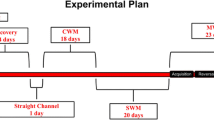
Neurocognitive impairment in response to methamphetamine (MA) has been proven in a variety of experimental and clinical studies. Elucidation of the underlying mechanisms of MA-induced cognitive deficits and finding preventive/therapeutic approaches need best-suited animal models. In modeling repeated MA exposure, while some believes that escalating doses simulate drug abuse conditions, others believe this regimen confers a preconditioning protection. The present study aimed to compare the effects of three different regimens of repeated MA administration on memory and cognitive function of adult rats. Rats in two different experimental groups were treated with escalating paradigms consisted of twice-daily i.p. injections; 1–4 mg/kg over 7 days or 1–10 mg/kg over 10 days. The third group received twice-daily doses of 15 mg/kg every other day over 14 days. Spatial working memory, novel object recognition task and anxiety-like behavior were measured sequentially in all MA-treated rats and vehicle-treated controls started from day 8 after last injection. All MA regimens decreased rates of spontaneous alternation in Y-maze and increased anxiety-like response. Short-term recognition memory was unchanged across all MA-treated animals, while long-term memory was impaired in the second and third MA regimen. Though MA deleterious effect especially in recognition memory is somehow dose dependent, preconditioning effect of increasing doses may be ruled out at least in the case of parameters measured here.





Similar content being viewed by others
References
Aggleton JP, Albasser MM, Aggleton DJ, Poirier GL, Pearce JM (2010) Lesions of the rat perirhinal cortex spare the acquisition of a complex configural visual discrimination yet impair object recognition. Behav Neurosci 124(1):55–68. doi:10.1037/a0018320
Ainge JA, van der Meer MA, Langston RF, Wood ER (2007) Exploring the role of context-dependent hippocampal activity in spatial alternation behavior. Hippocampus 17(10):988–1002
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13(2):93–110. doi:10.1007/s10339-011-0430-z
Belcher AM, O’Dell SJ, Marshall JF (2005) Impaired object recognition memory following methamphetamine, but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology 30(11):2026–2034
Bernheim A, See RE, Reichel CM (2016) Chronic methamphetamine self-administration disrupts cortical control of cognition. Neurosci Biobehav Rev 69:36–48. doi:10.1016/j.neubiorev.2016.07.020
Bisagno V, Ferguson D, Luine VN (2002) Short toxic methamphetamine schedule impairs object recognition task in male rats. Brain Res 940(1–2):95–101
Bizon JL, Foster TC, Alexander GE, Glisky EL (2012) Characterizing cognitive aging of working memory and executive function in animal models. Front Aging Neurosci 4:19 (eCollection 2012)
Bowyer JF, Hanig JP (2014) Amphetamine- and methamphetamine-induced hyperthermia: implications of the effects produced in brain vasculature and peripheral organs to forebrain neurotoxicity. Temperature (Austin) 1(3):172–182. doi:10.4161/23328940.2014.982049
Braren SH, Drapala D, Tulloch IK, Serrano PA (2014) Methamphetamine-induced short-term increase and long-term decrease in spatial working memory affects protein Kinase M zeta (PKMζ), dopamine, and glutamate receptors. Front Behav Neurosci 8:438. doi:10.3389/fnbeh.2014.00438
Cadet JL, Bisagno V (2016) Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front Psychiatry 6:189. doi:10.3389/fpsyt.2015.00189 (eCollection 2015)
Cadet JL, Brannock C, Ladenheim B, McCoy MT, Beauvais G, Hodges AB, Lehrmann E, Wood WH 3rd, Becker KG, Krasnova IN (2011) Methamphetamine preconditioning causes differential changes in striatal transcriptional responses to large doses of the drug. Dose Response 9(2):165–181
Clark RE, Kuczenski R, Segal DS (2007) Escalating dose, multiple binge methamphetamine regimen does not impair recognition memory in rats. Synapse 61(7):515–522
Darke S, Kaye S, McKetin R, Duflou J (2008) Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev 27(3):253–262. doi:10.1080/09595230801923702
Davidson C, Lee TH, Ellinwood EH (2005) Acute and chronic continuous methamphetamine have different long-term behavioral and neurochemical consequences. Neurochem Int 46(3):189–203
Dickerson BC, Eichenbaum H (2010) The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology 35(1):86–104. doi:10.1038/npp.2009.126
El Ayadi A, Zigmond MJ (2011) Low concentrations of methamphetamine can protect dopaminergic cells against a larger oxidative stress injury: mechanistic study. PLoS ONE 6(10):e24722
Ennaceur A (2010) One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res 215(2):244–254. doi:10.1016/j.bbr.2009.12.036
Ghazvini H, Khaksari M, Esmaeilpour K, Shabani M, Asadi-Shekaari M, Khodamoradi M, Sheibani V (2016) Effects of treatment with estrogen and progesterone on the methamphetamine-induced cognitive impairment in ovariectomized rats. Neurosci Lett 619:60–67. doi:10.1016/j.neulet.2016.02.057
Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson R, Methamphetamine Treatment Project Corporate Authors (2010) Anxiety disorders among methamphetamine dependent adults: association with post-treatment functioning. Am J Addict 19(5):385–390. doi:10.1111/j.1521-0391.2010.00061.x
Gonçalves J, Baptista S, Olesen MV, Fontes-Ribeiro C, Malva JO, Woldbye DP, Silva AP (2012) Methamphetamine-induced changes in the mice hippocampal neuropeptide Y system: implications for memory impairment. J Neurochem 123(6):1041–1053. doi:10.1111/jnc.12052
Gonzalez R, Bechara A, Martin EM (2007) Executive functions among individuals with methamphetamine or alcohol as drugs of choice: preliminary observations. J Clin Exp Neuropsychol 29(2):155–159
Grace CE, Schaefer TL, Herring NR, Graham DL, Skelton MR, Gudelsky GA, Williams MT, Vorhees CV (2010) Effect of a neurotoxic dose regimen of (+)-methamphetamine on behavior, plasma corticosterone, and brain monoamines in adult C57BL/6 mice. Neurotoxicol Teratol 32(3):346–355
Hajheidari S, Miladi-Gorji H, Bigdeli I (2015) Effect of the environmental enrichment on the severity of psychological dependence and voluntary methamphetamine consumption in methamphetamine withdrawn rats. Neurosci Lett 584:151–155. doi:10.1016/j.neulet.2014.10.017
Hart CL, Marvin CB, Silver R, Smith EE (2012) Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology 37(3):586–608
Hayase T, Yamamoto Y, Yamamoto K (2005) Persistent anxiogenic effects of a single or repeated doses of cocaine and methamphetamine: interactions with endogenous cannabinoid receptor ligands. Behav Pharmacol 16(5–6):395–404
He J, Yang Y, Yu Y, Li X, Li XM (2006) The effects of chronic administration of quetiapine on the methamphetamine-induced recognition memory impairment and dopaminergic terminal deficit in rats. Behav Brain Res 172(1):39–45
Hodges AB, Ladenheim B, McCoy MT, Beauvais G, Cai N, Krasnova IN, Cadet JL (2011) Long-term protective effects of methamphetamine preconditioning against single-day methamphetamine toxic challenges. Curr Neuropharmacol 9(1):35–39
Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH (2006) Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology 188(2):162–170
Hughes RN (2004) The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev 28(5):497–505
Kamei H, Nagai T, Nakano H, Togan Y, Takayanagi M, Takahashi K, Kobayashi K, Yoshida S, Maeda K, Takuma K, Nabeshima T, Yamada K (2006) Repeated methamphetamine treatment impairs recognition memory through a failure of novelty-induced ERK1/2 activation in the prefrontal cortex of mice. Biol Psychiatry 59(1):75–84
Kitanaka J, Kitanaka N, Takemura M (2008) Neurochemical consequences of dysphoric state during amphetamine withdrawal in animal models: a review. Neurochem Res 33(1):204–219 (Epub 2007 Jun 29)
Kitanaka N, Kitanaka J, Tatsuta T, Tanaka K, Watabe K, Nishiyama N, Morita Y, Takemura M (2010) Withdrawal from fixed-dose injection of methamphetamine decreases cerebral levels of 3-methoxy-4-hydroxyphenylglycol and induces the expression of anxiety-related behavior in mice. Neurochem Res 35(5):749–760. doi:10.1007/s11064-010-0132-4
Lee KW, Kim HC, Lee SY, Jang CG (2011) Methamphetamine-sensitized mice are accompanied by memory impairment and reduction of N-methyl-d-aspartate receptor ligand binding in the prefrontal cortex and hippocampus. Neuroscience 178:101–107. doi:10.1016/j.neuroscience.2011.01.025 (Epub 2011 Jan 19)
London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, Thompson PM, Brody AL, Geaga JA, Hong MS, Hayashi KM, Rawson RA, Ling W (2005) Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry 58(10):770–778
Madden LJ, Flynn CT, Zandonatti MA, May M, Parsons LH, Katner SN, Henriksen SJ, Fox HS (2005) Modeling human methamphetamine exposure in nonhuman primates: chronic dosing in the rhesus macaque leads to behavioral and physiological abnormalities. Neuropsychopharmacology 30(2):350–359
Marshall JF, Belcher AM, Feinstein EM, O’Dell SJ (2007) Methamphetamine-induced neural and cognitive changes in rodents. Addiction 102(Suppl 1):61–69
McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM (2005) The nature, time course and severity of methamphetamine withdrawal. Addiction 100(9):1320–1329
Miladi-Gorji H, Fadaei A, Bigdeli I (2015) Anxiety assessment in methamphetamine—sensitized and withdrawn rats: immediate and delayed effects. Iran J Psychiatry 10(3):150–157
North A, Swant J, Salvatore MF, Gamble-George J, Prins P, Butler B, Mittal MK, Heltsley R, Clark JT, Khoshbouei H (2013) Chronic methamphetamine exposure produces a delayed, long-lasting memory deficit. Synapse 67(5):245–257. doi:10.1002/syn.21635
Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA (2002) Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology 26(1):53–63
Pometlová M, Nohejlová-Deykun K, Slamberová R (2012) Anxiogenic effect of low-dose methamphetamine in the test of elevated plus-maze. Prague Med Rep 113(3):223–230
Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE (2011) Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology 36(4):782–792. doi:10.1038/npp.2010.212
Rusyniak DE (2013) Neurologic manifestations of chronic methamphetamine abuse. Psychiatr Clin N Am 36(2):261–275. doi:10.1016/j.psc.2013.02.005
Saito M, Terada M, Saito TR, Takahashi KW (1995) Effects of the long-term administration of methamphetamine on body weight, food intake, blood biochemistry and estrous cycle in rats. Exp Anim 43(5):747–754
Simões PF, Silva AP, Pereira FC, Marques E, Grade S, Milhazes N, Borges F, Ribeiro CF, Macedo TR (2007) Methamphetamine induces alterations on hippocampal NMDA and AMPA receptor subunit levels and impairs spatial working memory. Neuroscience 150(2):433–441
Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W (2000) Cognitive impairment in individuals currently using methamphetamine. Am J Addict 9(3):222–231
Simon SL, Dacey J, Glynn S, Rawson R, Ling W (2004) The effect of relapse on cognition in abstinent methamphetamine abusers. J Subst Abuse Treat 27(1):59–66
Šlamberová R, Pometlová M, Macúchová E, Nohejlová K, Stuchlík A, Valeš K (2015) Do the effects of prenatal exposure and acute treatment of methamphetamine on anxiety vary depending on the animal model used? Behav Brain Res 292:361–369. doi:10.1016/j.bbr.2015.07.001
Walf AA, Frye CA (2007) The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2(2):322–328
Woods SP, Rippeth JD, Conover E, Gongvatana A, Gonzalez R, Carey CL, Cherner M, Heaton RK, Grant I, HIV Neurobehavioral Research Center Group (2005) Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology 19(1):35–43
Yu S, Zhu L, Shen Q, Bai X, Di X (2015) Recent advances in methamphetamine neurotoxicity mechanisms and its molecular pathophysiology. Behav Neurol 2015:103969. doi:10.1155/2015/103969
Zhong N, Jiang H, Du J, Zhao Y, Sun H, Xu D, Li C, Zhuang W, Li X, Hashimoto K, Zhao M (2016) The cognitive impairments and psychological wellbeing of methamphetamine dependent patients compared with health controls. Prog Neuropsychopharmacol Biol Psychiatry 69:31–37. doi:10.1016/j.pnpbp.2016.04.005
Acknowledgements
The authors are thankful to the Neuroscience Research Center of Shahid Beheshti University of Medical Sciences for funding this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling editor: Maria Gulinello (Albert Einstein College of Medicine, New York).
Reviewers: Charles Vorhees (University of Cincinnati), Jun-Xu Li (University at Buffalo).
Rights and permissions
About this article
Cite this article
Seyedhosseini Tamijani, S.M., Beirami, E., Ahmadiani, A. et al. Effect of three different regimens of repeated methamphetamine on rats’ cognitive performance. Cogn Process 19, 107–115 (2018). https://doi.org/10.1007/s10339-017-0839-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10339-017-0839-0